生态环境学报 ›› 2025, Vol. 34 ›› Issue (4): 642-652.DOI: 10.16258/j.cnki.1674-5906.2025.04.013
陈岩1,2( ), 石成龙1,*(
), 石成龙1,*( ), 李璞君2, 肖江2,*(
), 李璞君2, 肖江2,*( ), 陈光才2
), 陈光才2
收稿日期:2024-10-20
出版日期:2025-04-18
发布日期:2025-04-24
通讯作者:
肖江。E-mail: jiangxiao0915@caf.ac.cn作者简介:陈岩(1998年生),男,硕士研究生,主要研究方向为环境功能材料制备及土壤重金属修复。E-mail: wellness1027@163.com
基金资助:
CHEN Yan1,2( ), SHI Chenglong1,*(
), SHI Chenglong1,*( ), LI Pujun2, XIAO Jiang2,*(
), LI Pujun2, XIAO Jiang2,*( ), CHEN Guangcai2
), CHEN Guangcai2
Received:2024-10-20
Online:2025-04-18
Published:2025-04-24
摘要: 前期研究以共水热炭化技术为支撑,建立植物修复衍生生物质/骨粉(PB/BM)水热体系有效地固定了PB中重金属于固相,但液相副产物(PB/BM-L)成分和潜在利用价值未深入剖析。该研究对PB/BM-L进行成分分析,并设置对照,1%添加量和3%添加量的处理组进行柳树盆栽试验评价其对碱性土壤及植物生长的影响,综合评估其潜在应用价值。结果表明,PB/BM-L中重金属Cd、Zn质量浓度均低于国家污水综合排放标准(GB 8978-1996,Cd≤0.1 mg∙L−1,Zn≤2 mg∙L−1);其富含氮(12.87-17.78 g∙L−1)、磷(13.13-20.03 g∙L−1)、有机碳(8.97-13.46 g∙L−1)等;pH处于酸性范围(4.53-4.72)且含有多种矿质元素,如Ca(26.61-42.85 g∙L−1)、Mg(5.28-9.33 g∙L−1)、Fe(5.28-9.33 g∙L−1)等;液相产物PB/BM-L主要有机组分依次为酚(52.81%)、酮(12.21%)、吡嗪(10.09%)、酯(8.64%)、醛(5.55%)、烃(3.62%)、酸(3.11%)、胺(2.81%)、醇(0.63%)、吡啶(0.53%)等。与对照组相比,盆栽试验中3%添加量的处理改善了土壤物理性质,降低了土壤pH(7.96-7.84),土壤含水量和孔隙度分别提高了2.01%和0.99%,而土壤容重降低了0.17 g∙cm−3,提高了土壤总氮(TN)、水解氮(HN)、总有机碳(TOC)、有效磷(AP)和速效钾(AK),同时土壤碱性磷酸酶(ALP)、蔗糖酶(Suc)、脲酶(Ure)活性分别提高了81.17%、293.04%、89.57%。苏柳J172(Salix×jiangsuensis cv. ‘J172’)的根、茎和叶生物量分别提高了(22.50%、26.17%、37.08%,p<0.05),且株高、茎粗分别增加了22.64%、19.23%。综上所述,PB/BM-L在碱性、贫瘠的土地上和植物生长改善方面具有良好的应用前景,有望实现废弃物的二次利用。
中图分类号:
陈岩, 石成龙, 李璞君, 肖江, 陈光才. 含重金属林木生物质与骨粉共水热液相产物:解析及应用潜力初步评价[J]. 生态环境学报, 2025, 34(4): 642-652.
CHEN Yan, SHI Chenglong, LI Pujun, XIAO Jiang, CHEN Guangcai. Co-hydrothermal Liquid Phase Product of Heavy Metal-containing Trees and Bone Meal: Analysis and Preliminary Evaluation[J]. Ecology and Environment, 2025, 34(4): 642-652.
| 实验顺序 | PB/BM混合比/ % | 反应温度/ ℃ | 固体负载量/ % | 停留时间/ min |
|---|---|---|---|---|
| PB/BM混合比 | *1) | 200 | 5 | 60 |
| 反应温度 | 25 | * | 5 | 60 |
| 固体负载量 | 25 | 220 | * | 60 |
| 停留时间 | 25 | 220 | 20 | * |
表1 单因素实验顺序及参数设定
Table 1 Single factor experiment sequence and parameters setting
| 实验顺序 | PB/BM混合比/ % | 反应温度/ ℃ | 固体负载量/ % | 停留时间/ min |
|---|---|---|---|---|
| PB/BM混合比 | *1) | 200 | 5 | 60 |
| 反应温度 | 25 | * | 5 | 60 |
| 固体负载量 | 25 | 220 | * | 60 |
| 停留时间 | 25 | 220 | 20 | * |
| 实验编号 | PB/BM混合比/% | 固体负载量/% | 时间/min |
|---|---|---|---|
| 1 | 22.2 | 14.3 | 60 |
| 2 | 28.6 | 14.3 | 60 |
| 3 | 22.2 | 20 | 60 |
| 4 | 28.6 | 20 | 60 |
| 5 | 22.2 | 17.15 | 45 |
| 6 | 28.6 | 17.15 | 45 |
| 7 | 22.2 | 17.15 | 75 |
| 8 | 28.6 | 17.15 | 75 |
| 9 | 25 | 14.3 | 45 |
| 10 | 25 | 20 | 45 |
| 11 | 25 | 14.3 | 75 |
| 12 | 25 | 20 | 75 |
| 13 | 25 | 17.15 | 60 |
| 14 | 25 | 17.15 | 60 |
| 15 | 25 | 17.15 | 60 |
| 16 | 25 | 17.15 | 60 |
| 17 | 25 | 17.15 | 60 |
表2 RSM Box-Behnken的设计方案
Table 2 The design scheme of the RSM Box-Behnken
| 实验编号 | PB/BM混合比/% | 固体负载量/% | 时间/min |
|---|---|---|---|
| 1 | 22.2 | 14.3 | 60 |
| 2 | 28.6 | 14.3 | 60 |
| 3 | 22.2 | 20 | 60 |
| 4 | 28.6 | 20 | 60 |
| 5 | 22.2 | 17.15 | 45 |
| 6 | 28.6 | 17.15 | 45 |
| 7 | 22.2 | 17.15 | 75 |
| 8 | 28.6 | 17.15 | 75 |
| 9 | 25 | 14.3 | 45 |
| 10 | 25 | 20 | 45 |
| 11 | 25 | 14.3 | 75 |
| 12 | 25 | 20 | 75 |
| 13 | 25 | 17.15 | 60 |
| 14 | 25 | 17.15 | 60 |
| 15 | 25 | 17.15 | 60 |
| 16 | 25 | 17.15 | 60 |
| 17 | 25 | 17.15 | 60 |
| 样品 | ρ(Mg)/ (g∙L−1) | ρ(Al)/ (g∙L−1) | ρ(Fe)/ (g∙L−1) | ρ(Cu)/ (g∙L−1) | ρ(Ca)/ (g∙L−1) | ρ(Na)/ (g∙L−1) |
|---|---|---|---|---|---|---|
| 1 | 6.23 | 0.045 | 0.54 | 1.0×10−4 | 26.61 | 63.22 |
| 2 | 5.77 | 0.048 | 0.29 | 8.5×10−5 | 27.92 | 61.99 |
| 3 | 9.33 | 0.065 | 0.82 | 1.1×10−4 | 42.85 | 94.23 |
| 4 | 8.00 | 0.062 | 0.59 | 1.0×10−4 | 40.80 | 90.25 |
| 5 | 7.23 | 0.053 | 0.54 | - | 32.41 | 81.35 |
| 6 | 6.59 | 0.061 | 0.65 | - | 36.20 | 81.16 |
| 7 | 7.47 | 0.054 | 0.26 | 8.0×10−5 | 36.86 | 81.25 |
| 8 | 7.21 | 0.052 | 0.41 | 1.2×10−4 | 36.63 | 78.16 |
| 9 | 5.28 | 0.028 | 0.28 | 2.0×10−5 | 29.01 | 66.02 |
| 10 | 7.82 | 0.047 | 0.60 | 7.0×10−5 | 43.75 | 98.61 |
| 11 | 6.10 | 0.035 | 0.15 | 2.0×10−4 | 29.14 | 66.75 |
| 12 | 8.70 | 0.058 | 0.60 | 7.0×10−5 | 42.00 | 89.08 |
| 13 | 5.95 | 0.037 | 0.23 | 1.9×10−4 | 30.48 | 67.37 |
| 14 | 6.18 | 0.021 | 0.46 | 1.1×10−3 | 31.94 | 73.58 |
| 15 | 5.85 | 0.025 | 0.27 | 5.0×10−5 | 28.67 | 66.78 |
| 16 | 5.75 | 0.034 | 0.21 | 2.2×10−4 | 29.17 | 69.58 |
| 17 | 6.31 | 0.034 | 0.26 | 6.5×10−5 | 31.13 | 73.51 |
表3 PB/BM-L中Mg、Al、Fe、Cu、Ca、Na质量
Table 3 Contents of Mg, Al, Fe, Cu, and Na in PB/BM-L
| 样品 | ρ(Mg)/ (g∙L−1) | ρ(Al)/ (g∙L−1) | ρ(Fe)/ (g∙L−1) | ρ(Cu)/ (g∙L−1) | ρ(Ca)/ (g∙L−1) | ρ(Na)/ (g∙L−1) |
|---|---|---|---|---|---|---|
| 1 | 6.23 | 0.045 | 0.54 | 1.0×10−4 | 26.61 | 63.22 |
| 2 | 5.77 | 0.048 | 0.29 | 8.5×10−5 | 27.92 | 61.99 |
| 3 | 9.33 | 0.065 | 0.82 | 1.1×10−4 | 42.85 | 94.23 |
| 4 | 8.00 | 0.062 | 0.59 | 1.0×10−4 | 40.80 | 90.25 |
| 5 | 7.23 | 0.053 | 0.54 | - | 32.41 | 81.35 |
| 6 | 6.59 | 0.061 | 0.65 | - | 36.20 | 81.16 |
| 7 | 7.47 | 0.054 | 0.26 | 8.0×10−5 | 36.86 | 81.25 |
| 8 | 7.21 | 0.052 | 0.41 | 1.2×10−4 | 36.63 | 78.16 |
| 9 | 5.28 | 0.028 | 0.28 | 2.0×10−5 | 29.01 | 66.02 |
| 10 | 7.82 | 0.047 | 0.60 | 7.0×10−5 | 43.75 | 98.61 |
| 11 | 6.10 | 0.035 | 0.15 | 2.0×10−4 | 29.14 | 66.75 |
| 12 | 8.70 | 0.058 | 0.60 | 7.0×10−5 | 42.00 | 89.08 |
| 13 | 5.95 | 0.037 | 0.23 | 1.9×10−4 | 30.48 | 67.37 |
| 14 | 6.18 | 0.021 | 0.46 | 1.1×10−3 | 31.94 | 73.58 |
| 15 | 5.85 | 0.025 | 0.27 | 5.0×10−5 | 28.67 | 66.78 |
| 16 | 5.75 | 0.034 | 0.21 | 2.2×10−4 | 29.17 | 69.58 |
| 17 | 6.31 | 0.034 | 0.26 | 6.5×10−5 | 31.13 | 73.51 |
| 样品 | ρ(TOC)/(g∙L−1) | ρ(N)/(g∙L−1) | ρ(P)/(g∙L−1) | ρ(K)/(g∙L−1) | pH |
|---|---|---|---|---|---|
| 1 | 9.07 | 12.87 | 0.32 | 13.50 | 4.53 |
| 2 | 8.97 | 13.60 | 0.29 | 13.19 | 4.62 |
| 3 | 12.64 | 15.82 | 0.42 | 20.03 | 4.61 |
| 4 | 12.79 | 16.25 | 0.34 | 19.53 | 4.62 |
| 5 | 11.72 | 16.00 | 0.28 | 17.03 | 4.61 |
| 6 | 11.59 | 16.71 | 0.37 | 16.63 | 4.70 |
| 7 | 11.84 | 13.83 | 0.25 | 17.22 | 4.55 |
| 8 | 11.95 | 16.21 | 0.32 | 16.69 | 4.60 |
| 9 | 9.58 | 14.63 | 0.27 | 12.95 | 4.72 |
| 10 | 13.32 | 17.78 | 0.34 | 19.41 | 4.67 |
| 11 | 9.72 | 12.39 | 0.22 | 13.77 | 4.58 |
| 12 | 13.46 | 14.99 | 0.33 | 19.59 | 4.58 |
| 13 | 9.95 | 13.82 | 0.29 | 13.72 | 4.65 |
| 14 | 10.01 | 13.87 | 0.26 | 14.42 | 4.67 |
| 15 | 9.69 | 13.50 | 0.27 | 13.13 | 4.62 |
| 16 | 10.12 | 13.79 | 0.26 | 13.33 | 4.61 |
| 17 | 9.80 | 14.36 | 0.26 | 14.51 | 4.60 |
表4 PB/BM-L中TN、TP、TK、TOC质量浓度和pH
Table 4 Mass concentrations of TN, TP, TK, TOC, and pH in PB/BM-L
| 样品 | ρ(TOC)/(g∙L−1) | ρ(N)/(g∙L−1) | ρ(P)/(g∙L−1) | ρ(K)/(g∙L−1) | pH |
|---|---|---|---|---|---|
| 1 | 9.07 | 12.87 | 0.32 | 13.50 | 4.53 |
| 2 | 8.97 | 13.60 | 0.29 | 13.19 | 4.62 |
| 3 | 12.64 | 15.82 | 0.42 | 20.03 | 4.61 |
| 4 | 12.79 | 16.25 | 0.34 | 19.53 | 4.62 |
| 5 | 11.72 | 16.00 | 0.28 | 17.03 | 4.61 |
| 6 | 11.59 | 16.71 | 0.37 | 16.63 | 4.70 |
| 7 | 11.84 | 13.83 | 0.25 | 17.22 | 4.55 |
| 8 | 11.95 | 16.21 | 0.32 | 16.69 | 4.60 |
| 9 | 9.58 | 14.63 | 0.27 | 12.95 | 4.72 |
| 10 | 13.32 | 17.78 | 0.34 | 19.41 | 4.67 |
| 11 | 9.72 | 12.39 | 0.22 | 13.77 | 4.58 |
| 12 | 13.46 | 14.99 | 0.33 | 19.59 | 4.58 |
| 13 | 9.95 | 13.82 | 0.29 | 13.72 | 4.65 |
| 14 | 10.01 | 13.87 | 0.26 | 14.42 | 4.67 |
| 15 | 9.69 | 13.50 | 0.27 | 13.13 | 4.62 |
| 16 | 10.12 | 13.79 | 0.26 | 13.33 | 4.61 |
| 17 | 9.80 | 14.36 | 0.26 | 14.51 | 4.60 |
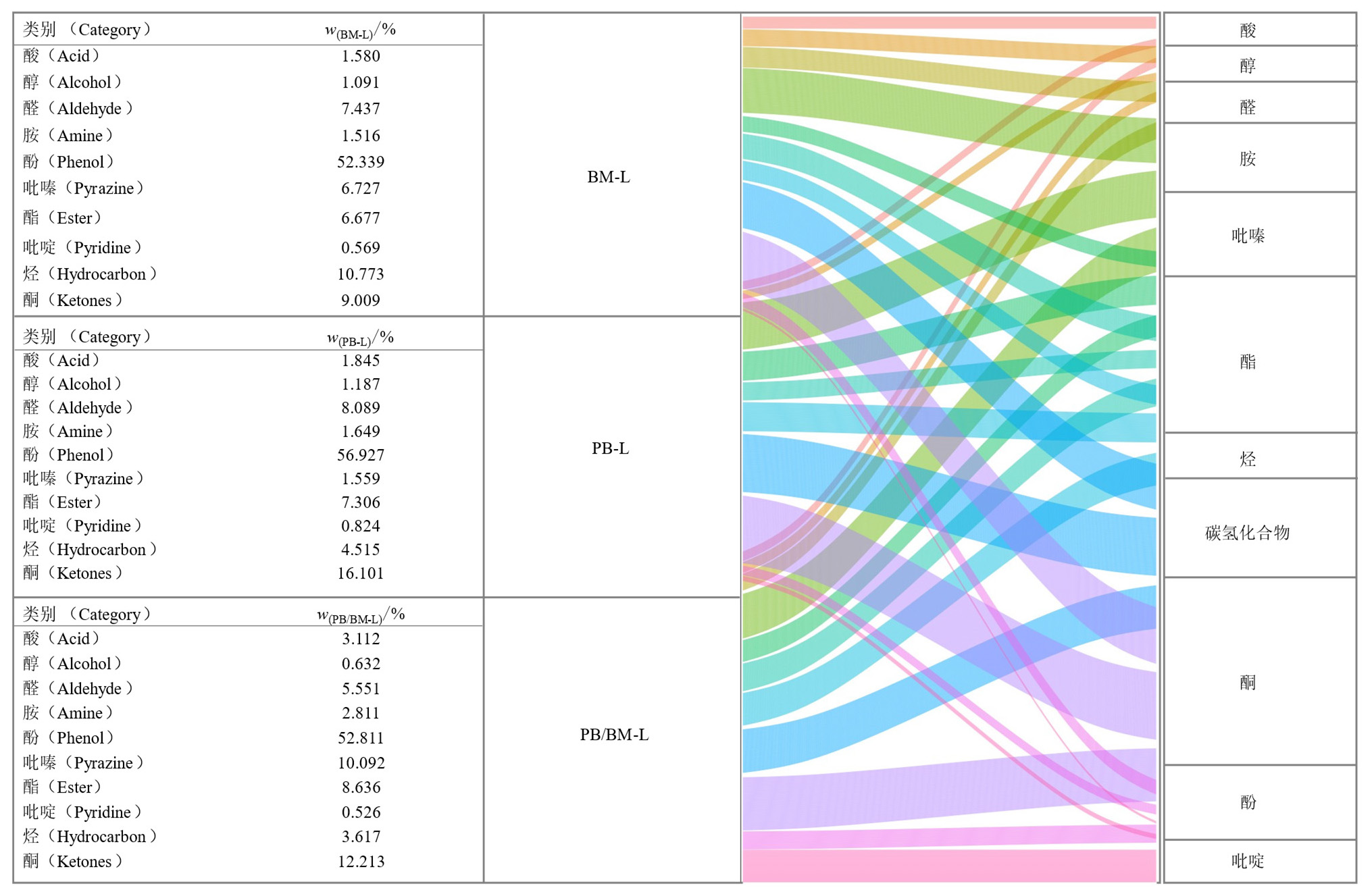
图2 PB-L、PB/BM-L和BM-L的有机物成分差异及相对含量桑基图
Figure 2 Differences and Sankey diagram in organic matter composition and relative content of PB-L, PB/BM-L, BM-L
| 处理 | 含水量/% | 容重/ (g∙cm−3) | 孔隙度/% | 土壤pH | 全氮质量分数/ (mg∙kg−1) | 水解氮质量分数/ (mg∙kg−1) | 有机碳质量分数/ (g∙kg−1) | 有效磷质量分数/ (mg∙kg−1) | 速效钾质量分数/ (mg∙kg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 15.79±0.15b | 2.07±0.01a | 37.78±0.35a | 7.96±0.03a | 3.03±0.03b | 236.30±1.56a | 27.69±0.30a | 31.39±1.12a | 49.07±6.06a | |
| L-1% | 16.04±0.45b | 1.98±0.01b | 37.23±0.64a | 7.93±0.01a | 3.07±0.02a | 238.59±1.29a | 27.75±0.48a | 32.23±0.69a | 59.21±2.92a | |
| L-3% | 17.80±0.22a | 1.90±0.01c | 38.77±0.73a | 7.84±0.03b | 3.12±0.01ab | 242.47±4.34a | 28.74±0.47a | 32.45±0.84a | 64.84±5.13a |
表5 不同处理下土壤理化性质
Table 5 The physical and chemical properties of soil under different treatments
| 处理 | 含水量/% | 容重/ (g∙cm−3) | 孔隙度/% | 土壤pH | 全氮质量分数/ (mg∙kg−1) | 水解氮质量分数/ (mg∙kg−1) | 有机碳质量分数/ (g∙kg−1) | 有效磷质量分数/ (mg∙kg−1) | 速效钾质量分数/ (mg∙kg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 15.79±0.15b | 2.07±0.01a | 37.78±0.35a | 7.96±0.03a | 3.03±0.03b | 236.30±1.56a | 27.69±0.30a | 31.39±1.12a | 49.07±6.06a | |
| L-1% | 16.04±0.45b | 1.98±0.01b | 37.23±0.64a | 7.93±0.01a | 3.07±0.02a | 238.59±1.29a | 27.75±0.48a | 32.23±0.69a | 59.21±2.92a | |
| L-3% | 17.80±0.22a | 1.90±0.01c | 38.77±0.73a | 7.84±0.03b | 3.12±0.01ab | 242.47±4.34a | 28.74±0.47a | 32.45±0.84a | 64.84±5.13a |
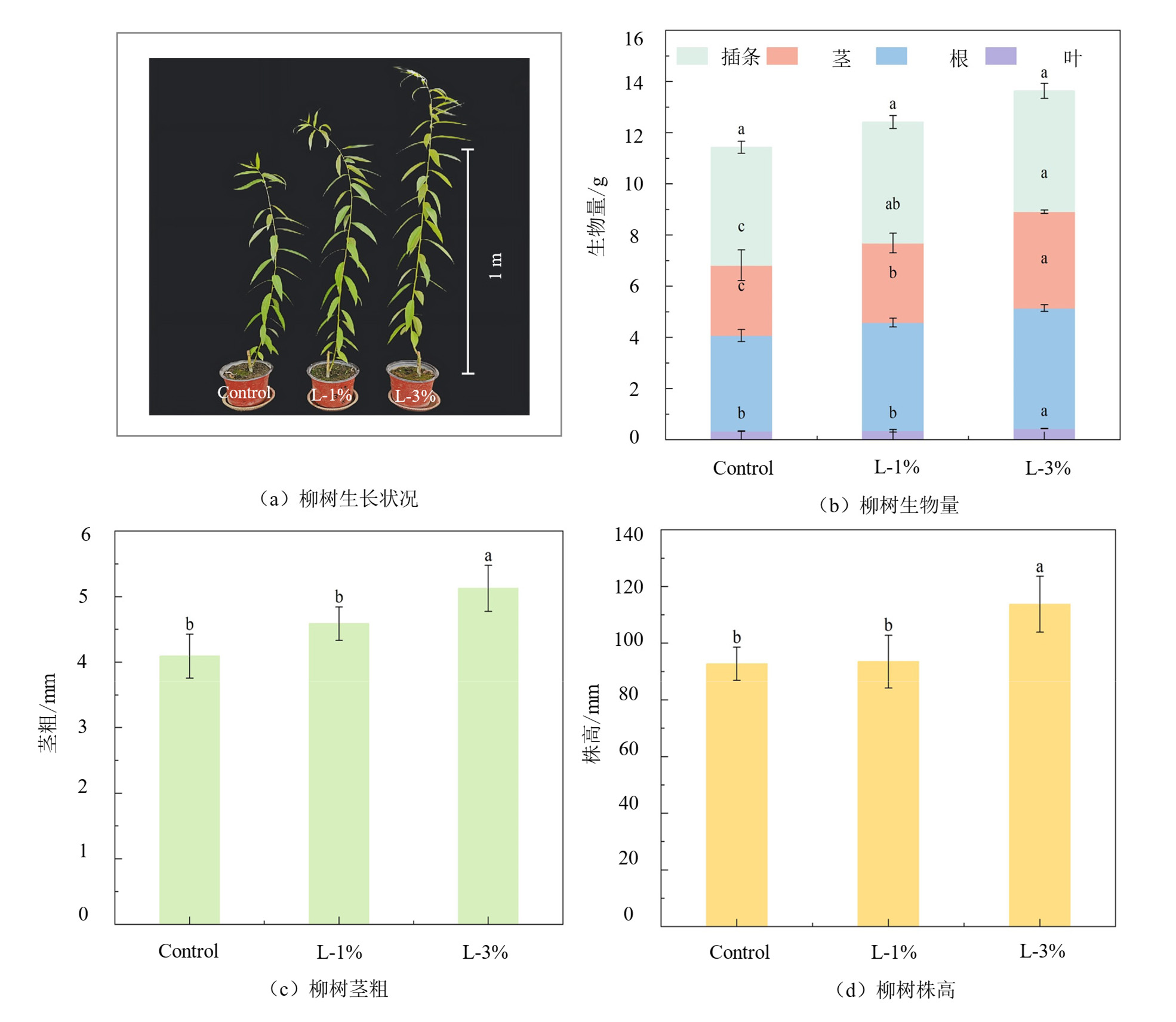
图5 苏柳J172在不同处理下的长势状况及株高、茎粗、生物量
Figure 5 Growth status, plant height, stem diameter and biomass of Salix×jiangsuensis cv.‘J172’ under different treatments
| [1] | AGEGNEHU G, SRIVASTAVA A K, BIRD M I, 2017. The role of biochar and biochar-compost in improving soil quality and crop performance: A review[J]. Applied Soil Ecology, 119: 156-170. |
| [2] | AKHTAR J, AMIN N A S, 2011. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass[J]. Renewable and Sustainable Energy Reviews, 15(3): 1615-1624. |
| [3] | BALASUNDRAM N, SUNDRAM K, SAMMAN S, 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses[J]. Food Chemistry, 99(1): 191-203. |
| [4] | BRAZDIS R I, FIERASCU I, AVRAMESCU S M, et al., 2021. Recent progress in the application of hydroxyapatite for the adsorption of heavy metals from water matrices[J]. Materials, 14(22): 6898. |
| [5] | DAHLAWI S, NAEEM A, RENGEL Z, et al., 2018. Biochar application for the remediation of salt-affected soils: Challenges and opportunities[J]. Science of the Total Environment, 625: 320-335. |
| [6] |
ELLIOTT D C, BILLER P, ROSS A B, et al., 2015. Hydrothermal liquefaction of biomass: Developments from batch to continuous process[J]. Bioresource Technology, 178: 147-156.
DOI PMID |
| [7] | FERNÁNDEZ-DELGADO M, DELAMO-MATEOS E, LUCAS S, et al., 2022. Liquid fertilizer production from organic waste by conventional and microwave-assisted extraction technologies: Techno-economic and environmental assessment[J]. Science of the Total Environment, 806(Part 4): 150904. |
| [8] | GAI C, ZHANG Y H, CHEN W T, et al., 2014. Energy and nutrient recovery efficiencies in biocrude oil produced via hydrothermal liquefaction of Chlorella pyrenoidosa[J]. RSC Advances, 4(33): 16958-16967. |
| [9] | GARBOWSKI T, BAR-MICHALCZYK D, CHARAZIŃSKA S, et al., 2023. An overview of natural soil amendments in agriculture[J]. Soil and Tillage Research, 225: 105462. |
| [10] | GOLLAKOTA A R K, KISHORE N, GU S, 2018. A review on hydrothermal liquefaction of biomass[J]. Renewable and Sustainable Energy Reviews, 81(Part 1): 1378-1392. |
| [11] | GUGLIUCCI W, CIRILLO V, MAGGIO A, et al., 2023. Valorisation of hydrothermal liquefaction wastewater in agriculture: Effects on tobacco plants and rhizosphere microbiota[J]. Frontiers in Plant Science, 14: 1180061. |
| [12] | HE C, GIANNIS A, WANG J Y, 2013. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior[J]. Applied Energy, 111: 257-266. |
| [13] | HE K, HE G, WANG C P, et al., 2020. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil[J]. Applied Soil Ecology, 155: 103674. |
| [14] | HUNT J, DUPONTE M, SATO D, et al., 2010. The basics of biochar: A natural soil amendment[J]. Soil and Crop Management, 30(7): 1-6. |
| [15] | KLOSS S, ZEHETNER F, WIMMER B, et al., 2014. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions[J]. Journal of Plant Nutrition and Soil Science, 177(1): 3-15. |
| [16] | KREY T, VASSILEV N, BAUM C, et al., 2013. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions[J]. European Journal of Soil Biology, 55: 124-130. |
| [17] | LENG L J, ZHANG W J, PENG H Y, et al., 2020. Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: A review[J]. Chemical Engineering Journal, 401: 126030. |
| [18] |
LEÓN M, MARCILLA A F, GARCÍA Á N, 2019. Hydrothermal liquefaction (HTL) of animal by-products: Influence of operating conditions[J]. Waste Management, 99: 49-59.
DOI PMID |
| [19] | LI C F, ZHOU K H, QIN W Q, et al., 2019. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques[J]. Soil and Sediment Contamination: An International Journal, 28(4): 380-394. |
| [20] | MADDI B, PANISKO E, WIETSMA T, et al., 2016. Quantitative characterization of the aqueous fraction from hydrothermal liquefaction of algae[J]. Biomass and Bioenergy, 93: 122-130. |
| [21] | MADSEN R B, BILLER P, JENSEN M M, et al., 2016. Predicting the chemical composition of aqueous phase from hydrothermal liquefaction of model compounds and biomasses[J]. Energy & Fuels, 30(12): 10470-10483. |
| [22] | PAN X, SHI M, CHEN X C, et al., 2022. An investigation into biochar, acid-modified biochar, and wood vinegar on the remediation of saline-alkali soil and the growth of strawberries[J]. Frontiers in Environmental Science, 10: 1057384. |
| [23] | PAULINE A L, JOSEPH K, 2020. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel-A review of mechanisms and process parameters[J]. Fuel, 279: 118472. |
| [24] | PETROVIĆ J, ERCEGOVIĆ M, SIMIĆ M, et al., 2024. Hydrothermal carbonization of waste biomass: A review of hydrochar preparation and environmental application[J]. Processes, 12(1): 207. |
| [25] | PETROVIĿ J, PERIŠIĿ N, MAKSIMOVIĿ J D Ŀ, et al., 2016. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase[J]. Journal of Analytical and Applied Pyrolysis, 118: 267-277. |
| [26] |
REGMI P, MOSCOSO J L G, KUMAR S, et al., 2012. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process[J]. Journal of Environmental Management, 109: 61-69.
DOI PMID |
| [27] | SUNDAR RAJAN P, GOPINAATH K P, ARUN J, et al., 2021. Insights into valuing the aqueous phase derived from hydrothermal liquefaction[J]. Renewable and Sustainable Energy Reviews, 144: 111019. |
| [28] | TIAN K, ZHAO Y C, XU X H, et al., 2015. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis[J]. Agriculture, Ecosystems & Environment, 204: 40-50. |
| [29] | VALDEZ P J, NELSON M C, WANG H Y, et al., 2012. Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions[J]. Biomass and Bioenergy, 46: 317-331. |
| [30] | WANG R K, LIN Z H, MENG S, et al., 2022. Effect of lignocellulosic components on the hydrothermal carbonization reaction pathway and product properties of protein[J]. Energy, 259: 125063. |
| [31] | XIAO J, HU R, CHEN G C, 2020. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd (II), Cu (II) and Pb (II)[J]. Journal of Hazardous Materials, 387: 121980. |
| [32] | XU G, ZHANG Y, SUN J N, et al., 2016. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil[J]. Science of the Total Environment, 568: 910-915. |
| [33] | YANG C, WANG S Z, REN M M, et al., 2019. Hydrothermal liquefaction of an animal carcass for biocrude oil[J]. Energy & Fuels, 33(11): 11302-11309. |
| [34] |
YUAN T, CHENG Y F, HUANG W W, et al., 2018. Fertilizer potential of liquid product from hydrothermal treatment of swine manure[J]. Waste Management, 77: 166-171.
DOI PMID |
| [35] | ZHANG X H, JIANG W K, MA H, et al., 2020. Relationship between the formation of oligomers and monophenols and lignin structure during pyrolysis process[J]. Fuel, 276: 118048. |
| [36] | ZHOU C L, SONG X, WANG Y W, et al., 2022. The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil[J]. Chemosphere, 286(Part 3): 131810. |
| [37] | ZHU Z, ROSENDAHL L, TOOR S S, et al., 2015. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation[J]. Applied Energy, 137: 183-192. |
| [38] | 鲍士旦, 2000. 土壤农化分析[M]. 第3版. 北京: 中国农业出版社. |
| BAO S D, 2000. Soil agricultural chemistry analysis[M]. Third edition. Beijing: China Agriculture Press. | |
| [39] | 林开敏, 叶发茂, 林艳, 等, 2010. 酚类物质对土壤和植物的作用机制研究进展[J]. 中国生态农业学报, 18(5): 1130-1137. |
| LIN K M, YE F M, LIN Y, et al., 2010. Research advances of phenolic functional mechanisms in soils and plants[J]. Chinese Journal of Eco-Agriculture, 18(5): 1130-1137. | |
| [40] | 关松荫, 1986. 土壤酶及其研究法[M]. 北京: 农业出版社. |
| GUAN S Y, 1986. Soil enzymes and their research methods[M]. Beijing: Agriculture Press. | |
| [41] | 刘凯楠, 李国峰, 云梁, 等, 2023. 农业废弃生物质在废水处理中的应用研究进展[J]. 合成材料老化与应用, 52(3): 119-121. |
| LIU K N, LI G F, YUN L, et al., 2023. Research Progress on the Application of Agricultural Waste Biomass in Wastewater Treatment[J]. Synthetic Materials Aging and Application, 52(3): 119-121. | |
| [42] | 马云华, 王秀峰, 魏珉, 等, 2005. 黄瓜连作土壤酚酸类物质积累对土壤微生物和酶活性的影响[J]. 应用生态学报, 16(11): 145-149. |
| MA Y H, WANG X F, WEI M, et al., 2005. Accumulation of phenolic acids in continuously cropped cucumber soil and their effects on soil microbes and enzyme activities[J]. Chinese Journal of Applied Ecology, 16(11): 145-149. | |
| [43] | 欧祥鹏, 郑永红, 张治国, 等, 2024. 羟基磷灰石在土壤污染修复中的应用研究进展[J]. 应用化工, 53(6): 1412-1415. |
| OU X P, ZHENG Y H, ZHANG Z G, et al., 2024. Research progress on the application of hydroxyapatite in soil pollution remediation[J]. Applied Chemical Industry, 53(6): 1412-1415. | |
| [44] |
徐永洞, 刘志丹, 2021. 生物质水热液化水相产物形成机理及资源回收[J]. 化学进展, 33(11): 2150-2162.
DOI |
| XU Y D, LIU Z D, 2021. Formation mechanism and resource recovery perspectives of aqueous phase from hydrothermal liquefaction of biomass[J]. Progress Chemistry, 33(11): 2150-2162. | |
| [45] | 杨家学, 高微微, 2009. 酚酸类化感物质对两种西洋参病原真菌的作用[J]. 中国农学通报, 25(9): 207-211. |
| YANG J X, GAO W W, 2009. Effects of phenolic allelochemicals on the pathogen of Panax quiquefolium L.[J]. Chinese Agricultural Science Bulletin, 25(9): 207-211. | |
| [46] | 王友保, 2018. 土壤污染生态修复实验技术[M]. 北京: 科学出版社. |
| WANG Y B, 2018. Experimental technology for ecological remediation of soil pollution[M]. Beijing: Science Press. | |
| [47] |
吴东阳, 吴家欢, 李伟志, 等, 2024. 蚓粪、猪粪配施化肥对土壤质量、辣椒生长及品质的影响[J]. 生态环境学报, 33(9): 1416-1425.
DOI |
| WU D Y, WU J H, LI W Z, et al., 2024. Effects of vermicompost and pig manure combined with chemical fertilizers on soil quality, growth and quality of peppers[J]. Ecology and Environmental Sciences, 33(9): 1416-1425. | |
| [48] | 中华人民共和国环境保护部, 1997. 中华人民共和国国家标准污水综合排放标准: GB 8978—1996[S]. 北京: 中国环境出版社: 9-12. |
| Ministry of Environmental Protection of the People’s Republic of China, 1997. National standard of the People’s Republic of China integrated wastewater discharge standard: GB 8978—1996[S]. Beijing: China Environmental Press. | |
| [49] | 中华人民共和国环境保护部, 2007. 固体废物浸出毒性浸出方法醋酸缓冲溶液法: HJ/T 300—2007[S]. 北京: 中国环境出版社: 4-13. |
| Ministry of Environmental Protection of the People’s Republic of China, 2007. Solid waste Extraction procedure for leaching toxicity Acetic acid buffer solution method: HJ/T 300—2007[S]. Beijing: China Environmental Press: 4-13. |
| [1] | 陈琳, 兰冠宇, 徐妍, 李雪, 毛雪飞. 氢键有机框架材料在环境污染物吸附和检测中的研究进展[J]. 生态环境学报, 2025, 34(3): 474-483. |
| [2] | 王斌, 曾兆荷, 董璐, 岳林. 内梅罗指数法在地下水水质评价中的修正探讨与实践效果[J]. 生态环境学报, 2025, 34(2): 293-301. |
| [3] | 张传华, 刘力, 代杰, 李曼曼, 张凤太, 邓凌. 基于土壤重金属污染和累积性评价的耕地环境质量类别划分与风险管控[J]. 生态环境学报, 2025, 34(2): 311-320. |
| [4] | 韩军超, 郑茂坤, 涂晨, 刘颖, 曹振宇, 邢倩雯, 申卫收, 骆永明. 趋磁细菌在环境污染修复中的应用研究进展与展望[J]. 生态环境学报, 2025, 34(1): 145-155. |
| [5] | 常春英, 王刚, 曹浩轩, 邓一荣, 陶亮. 模拟干湿过程对稳定化修复土壤中重金属Ni和Pb的影响[J]. 生态环境学报, 2025, 34(1): 118-125. |
| [6] | 丛鑫, 张怀迪, 张荣, 赵琛, 陈坤, 刘寒冰. 基于Meta分析的近10年中国农田土壤重金属污染特征与风险解析[J]. 生态环境学报, 2024, 33(9): 1451-1459. |
| [7] | 刘东宜, 屈永华, 冯耀伟, 屈冉. 基于网格搜索优化CatBoost模型的GF-5卫星影像铬离子含量反演研究[J]. 生态环境学报, 2024, 33(9): 1460-1470. |
| [8] | 欧阳美凤, 尹宇莹, 张金谌, 刘清霖, 谢意南, 方平. 洞庭湖典型水域重金属含量的空间分布与来源解析[J]. 生态环境学报, 2024, 33(8): 1269-1278. |
| [9] | 吴文伟, 沈城, 沙晨燕, 林匡飞, 吴健, 谢雨晴, 周璇. 城市工业地块土壤重金属污染风险评价与源解析[J]. 生态环境学报, 2024, 33(5): 791-801. |
| [10] | 肖江, 李晓刚, 赵博, 陈岩, 陈光才. 微纳富磷生物炭对土壤-苏柳系统中Cu和Pb稳定性的影响[J]. 生态环境学报, 2024, 33(3): 439-449. |
| [11] | 江润海, 温绍福, 朱城强, 张梅, 杨润玲, 王春雪, 侯秀丽. 铅污染矿区中耐铅解磷菌对玉米的促生及根际铅的固化效应[J]. 生态环境学报, 2024, 33(2): 291-300. |
| [12] | 李嘉惠, 童辉, 陈曼佳, 刘承帅, 姜琪, 易秀. 微氧生物亚铁氧化及其重金属固定效应研究进展[J]. 生态环境学报, 2024, 33(2): 310-320. |
| [13] | 马志伟, 张丛志, 赵占辉, 吴其聪, 赵金花, 陈卓, 李敬王, 张楠, 薛雅, 王娅茹, 陆芸萱, 张佳宝. 基于木本泥炭的土壤健康培育研究进展[J]. 生态环境学报, 2024, 33(12): 1964-1977. |
| [14] | 李璞君, 唐丽, 赵博, 邸东柳, 陈岩, 肖江, 陈光才. 生物炭基土壤改良剂对锑矿区土壤质量及亮叶桦生长的影响[J]. 生态环境学报, 2024, 33(12): 1953-1963. |
| [15] | 唐舒娅, 王春辉, 宋靖, 李刚. 环象山港区域土壤重金属污染特征及风险评估[J]. 生态环境学报, 2024, 33(11): 1768-1781. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
