生态环境学报 ›› 2024, Vol. 33 ›› Issue (2): 310-320.DOI: 10.16258/j.cnki.1674-5906.2024.02.015
李嘉惠1( ), 童辉2,3,*(
), 童辉2,3,*( ), 陈曼佳2, 刘承帅3, 姜琪2, 易秀1,*(
), 陈曼佳2, 刘承帅3, 姜琪2, 易秀1,*( )
)
收稿日期:2023-09-20
出版日期:2024-02-18
发布日期:2024-04-03
通讯作者:
易秀。E-mail: yixiu@chd.edu.cn作者简介:李嘉惠(1999年生),女,硕士研究生,主要从事湿地土壤铁氧化物的环境效应。E-mail: 2021129064@chd.edu.cn
基金资助:
LI Jiahui1( ), TONG Hui2,3,*(
), TONG Hui2,3,*( ), CHEN Manjia2, LIU Chengshuai3, JIANG Qi2, YI Xiu1,*(
), CHEN Manjia2, LIU Chengshuai3, JIANG Qi2, YI Xiu1,*( )
)
Received:2023-09-20
Online:2024-02-18
Published:2024-04-03
摘要:
铁的生物地球化学循环对于多种环境过程至关重要,如碳封存、温室气体排放以及营养元素和有毒金属的迁移和转化。近年来,随着分离培养方式及分子生物学方法的发展,作为铁循环的重要组成部分的微氧生物铁氧化的研究取得了显著的进展。微氧型亚铁氧化菌广泛分布于近中性环境中,其分离栖息地从地下水、湿地、溪流延展至深海环境。微氧生物亚铁氧化成矿过程主要发生在细胞表面,生成比表面积较大的无定型铁氧化物。大部分微氧型亚铁氧化菌通过形成鞘状或螺旋柄状结构的胞外多聚物吸附生成的铁氧化物,防止自身被铁氧化物包埋,导致无法正常代谢而死亡。亚铁氧化成矿过程可吸附和共沉淀重金属元素,降低重金属的移动性和生物可利用性,从而缓解重金属的污染,为治理环境污染提供新的思路。文章主要总结了近年来国内外对嗜中性微氧型亚铁氧化菌的研究进展,包括其代谢特征、种类及分布、以及亚铁氧化菌的成矿机制和成矿过程对重金属迁移转化的影响。最后对如何快速有效地分离微氧型亚铁氧化菌、明确成矿过程中的特殊结构的形成机制等问题进行了讨论和展望。
中图分类号:
李嘉惠, 童辉, 陈曼佳, 刘承帅, 姜琪, 易秀. 微氧生物亚铁氧化及其重金属固定效应研究进展[J]. 生态环境学报, 2024, 33(2): 310-320.
LI Jiahui, TONG Hui, CHEN Manjia, LIU Chengshuai, JIANG Qi, YI Xiu. Formation of Fe(Ⅲ) Minerals by Microaerophilic Fe(Ⅱ)-oxidizing Bacteria and Its Effect on Immobilization of Heavy Metals: A Review[J]. Ecology and Environment, 2024, 33(2): 310-320.
| 样品类型 | 来源 | pH | O2浓度/ (μmol∙L−1) | Fe(II)浓度/ (μmol∙L−1) | 氧化速率比 (生物: 非生物) | 培养环境 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 纯培养 | Sideroxydans paludicola BrT | 6.5 | 2.5‒5 | 10‒100 | 1.4‒2.1 | 实验室中长期培养 | Neubauer et al., |
| 纯培养 | Sideroxydans lithotrophicus | 6.2 | 9‒50 | 25 | 1.6 | 利用电化学方法监测 | Druschel et al., |
| 纯培养 | Mariprofundus ferrooxydans | - | ~80 | 0.34 | >1 | 监测阴极电流的变化 | Summers et al., |
| 纯培养 | Sideroxydans spp. | 6.8 | 1‒30 | 550‒800 | <1 | 控制不同条件培养 | Maisch et al., |
| 铁絮环境 | 含铁地下水渗出处 | 6.8‒7.1 | 5‒40 | 60‒120 | 1.8‒4.9 | 原位分析 | Emerson et al., |
| 铁絮环境 | 含铁地下水渗出处 | - | - | 59 | 2.6 | 控制铁的浓度 | James et al., |
| 铁絮环境 | 含铁絮的溪水 | 6.35 | 38‒290 | 116 | 1.4‒4 | 未控制氧气浓度 | Rentz et al., |
| 铁絮环境 | 含铁絮的温泉及湖泊 | 5.7‒7.5 | 3.4‒343 | 0.2‒166 | 0.8‒8.9 | 原位分析结合实验培养 | ST Clair et al., |
表1 不同环境中亚铁氧化动力学
Table 1 Summary of studies on Fe(II) oxidation kinetics under micro-aerobic conditions
| 样品类型 | 来源 | pH | O2浓度/ (μmol∙L−1) | Fe(II)浓度/ (μmol∙L−1) | 氧化速率比 (生物: 非生物) | 培养环境 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 纯培养 | Sideroxydans paludicola BrT | 6.5 | 2.5‒5 | 10‒100 | 1.4‒2.1 | 实验室中长期培养 | Neubauer et al., |
| 纯培养 | Sideroxydans lithotrophicus | 6.2 | 9‒50 | 25 | 1.6 | 利用电化学方法监测 | Druschel et al., |
| 纯培养 | Mariprofundus ferrooxydans | - | ~80 | 0.34 | >1 | 监测阴极电流的变化 | Summers et al., |
| 纯培养 | Sideroxydans spp. | 6.8 | 1‒30 | 550‒800 | <1 | 控制不同条件培养 | Maisch et al., |
| 铁絮环境 | 含铁地下水渗出处 | 6.8‒7.1 | 5‒40 | 60‒120 | 1.8‒4.9 | 原位分析 | Emerson et al., |
| 铁絮环境 | 含铁地下水渗出处 | - | - | 59 | 2.6 | 控制铁的浓度 | James et al., |
| 铁絮环境 | 含铁絮的溪水 | 6.35 | 38‒290 | 116 | 1.4‒4 | 未控制氧气浓度 | Rentz et al., |
| 铁絮环境 | 含铁絮的温泉及湖泊 | 5.7‒7.5 | 3.4‒343 | 0.2‒166 | 0.8‒8.9 | 原位分析结合实验培养 | ST Clair et al., |
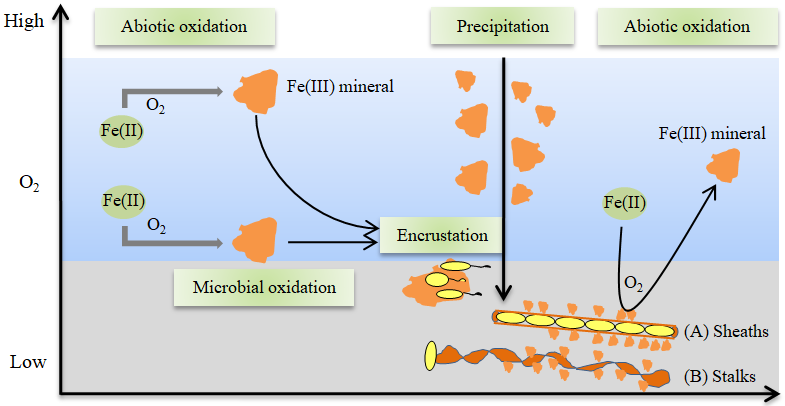
图1 有氧环境中铁的化学和生物氧化作用 图中,黄色椭圆形表示微氧型亚铁氧化菌,棕黄色不规则形状表示铁氧化物;(A)和(B)表示形成的鞘状和螺旋杆状特殊结构
Figure 1 The chemical and microbial Fe(II) oxidation under aerobic conditions
| 分类 | 微生物 | GenBank号 | 形成柄/鞘 | 来源 | 参考文献 |
|---|---|---|---|---|---|
| Alphaproteobacteria | Bradyrhizobium sp.22 | 563438928 | 否 | 地下水, 美国 | Benzine et al., |
| Bradyrhizobium sp.wssl4 | 381351228 | 否 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Dechlorospirillum sp. M1 | 255709709 | 否 | 含有铁氧化物的淡水溪流, 美国 | Picardal et al., | |
| Ochrobactrumsp. EEELCW01 | 2696486 | 否 | 砷污染的根际水稻土, 中国 | Luo et al., | |
| Betaproteobacteria | Beta proteobacterium TW2 | 20799634 | 否 | 淡水沉积物, 美国 | Sobolev et al., |
| Cupriavidus necatar ss1-6-6 | 563438932 | 否 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Ferrigenium kumadai An22 | 894215544 | 否 | 水稻土, 日本 | Khalifa et al., | |
| Ferriphaselus amincola | 387762392 | 是 | 地下水, 日本 | Kato et al., | |
| Ferritrophicum radicicola CCJ | 90992749 | 否 | 酸性矿山废水周围的根际土, 美国 | Weiss et al., | |
| Gallionella ferruginea | 1171632 | 是 | 饮用水井, 60 m深, 瑞典 | Hallbeck et al., | |
| Gallionella sp. R-1 | 343175302 | 是 | 富铁地下水温泉, 美国 | Krepski et al., | |
| Leptothrix cholodnii SP-6 | 444304199 | 是 | 排水管道周围的絮状物, 美国 | Siering et al., | |
| Leptothrix ochracea SCGC | 310706513 | 是 | 水渗出地表周围形成的矿物, 美国 | Fleming et al., | |
| Ralstonia solanacearum in4ss52 | 381351227 | 是 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Sideroxyarcus emersonii | 2764705 | 是 | 湿地土壤, 日本 | Kato et al., | |
| Sideroxydans lithotrophicus ES-1 | 444439416 | 否 | 地下水渗出处, 美国 | Emerson et al., | |
| Sideroxydans paludicola BrT | 90992751 | 是 | 湿地根际周围, 美国 | Weiss et al., | |
| Zetaproteobacteria | Mariprofundus erugo strain P3 | 11634928 | 是 | 河流入河口, 美国 | Garrison et al., |
| Mariprofundus ferrooxydans GSB2 | 314909558 | 是 | 海湾中富铁的絮状物, 美国 | McBeth et al., | |
| Mariprofundus ferrooxydans PV-1 | 145226685 | 是 | 深海热液, 美国 | Singer et al., | |
| Mariprofundus micogutta ET2 | 1093294039 | 是 | 深海沉积物, 日本 | Makita et al., | |
| Unclassified | LT575232 | 是 | 滨海沉积物, 丹麦 | Laufer et al., |
表2 已分离培养的微氧型亚铁氧化菌
Table 2 Representative neutrophilic, microaerophilic FeOB from different environments
| 分类 | 微生物 | GenBank号 | 形成柄/鞘 | 来源 | 参考文献 |
|---|---|---|---|---|---|
| Alphaproteobacteria | Bradyrhizobium sp.22 | 563438928 | 否 | 地下水, 美国 | Benzine et al., |
| Bradyrhizobium sp.wssl4 | 381351228 | 否 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Dechlorospirillum sp. M1 | 255709709 | 否 | 含有铁氧化物的淡水溪流, 美国 | Picardal et al., | |
| Ochrobactrumsp. EEELCW01 | 2696486 | 否 | 砷污染的根际水稻土, 中国 | Luo et al., | |
| Betaproteobacteria | Beta proteobacterium TW2 | 20799634 | 否 | 淡水沉积物, 美国 | Sobolev et al., |
| Cupriavidus necatar ss1-6-6 | 563438932 | 否 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Ferrigenium kumadai An22 | 894215544 | 否 | 水稻土, 日本 | Khalifa et al., | |
| Ferriphaselus amincola | 387762392 | 是 | 地下水, 日本 | Kato et al., | |
| Ferritrophicum radicicola CCJ | 90992749 | 否 | 酸性矿山废水周围的根际土, 美国 | Weiss et al., | |
| Gallionella ferruginea | 1171632 | 是 | 饮用水井, 60 m深, 瑞典 | Hallbeck et al., | |
| Gallionella sp. R-1 | 343175302 | 是 | 富铁地下水温泉, 美国 | Krepski et al., | |
| Leptothrix cholodnii SP-6 | 444304199 | 是 | 排水管道周围的絮状物, 美国 | Siering et al., | |
| Leptothrix ochracea SCGC | 310706513 | 是 | 水渗出地表周围形成的矿物, 美国 | Fleming et al., | |
| Ralstonia solanacearum in4ss52 | 381351227 | 是 | 湿地土壤、地下水, 美国 | Shelobolina et al., | |
| Sideroxyarcus emersonii | 2764705 | 是 | 湿地土壤, 日本 | Kato et al., | |
| Sideroxydans lithotrophicus ES-1 | 444439416 | 否 | 地下水渗出处, 美国 | Emerson et al., | |
| Sideroxydans paludicola BrT | 90992751 | 是 | 湿地根际周围, 美国 | Weiss et al., | |
| Zetaproteobacteria | Mariprofundus erugo strain P3 | 11634928 | 是 | 河流入河口, 美国 | Garrison et al., |
| Mariprofundus ferrooxydans GSB2 | 314909558 | 是 | 海湾中富铁的絮状物, 美国 | McBeth et al., | |
| Mariprofundus ferrooxydans PV-1 | 145226685 | 是 | 深海热液, 美国 | Singer et al., | |
| Mariprofundus micogutta ET2 | 1093294039 | 是 | 深海沉积物, 日本 | Makita et al., | |
| Unclassified | LT575232 | 是 | 滨海沉积物, 丹麦 | Laufer et al., |
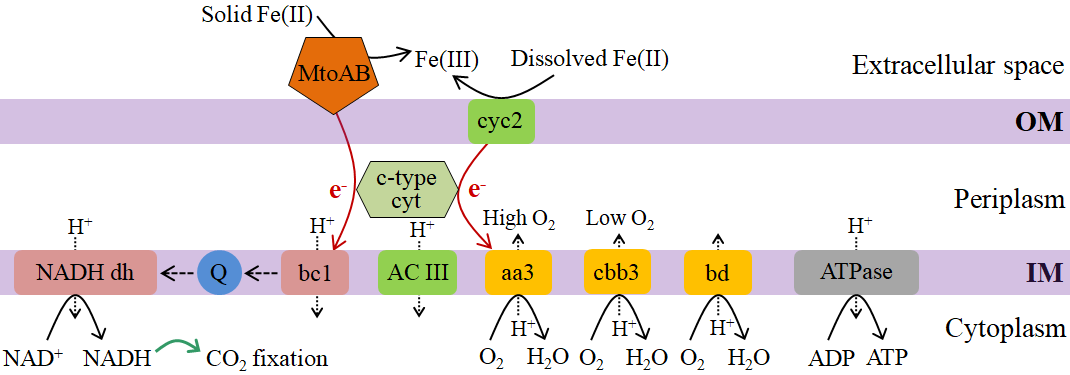
图2 微氧型亚铁氧化菌驱动的铁氧化过程及电子传递示意图 该图来源于下列文献: Emerson et al., 2013; Kato et al., 2015; Mühling et al., 2016; Blackwell et al., 2020; Chen et al., 2022; Zhou et al., 2022b
Figure 2 Schematic of Fe(II) oxidation and electron transport driven by microaerophilic FeOB OM: outer membrane; IM: inner membrane; NADH dh: NAD dehydrogenase; Q: quinonepool; cyc: cytochrome c; bc1: cytochrome bc1 complex; ACIII: alternative complex III; aa3: aa3-type cytochrome c oxidase; cbb3: cbb3-type cytochrome c oxidase; bd: cytochrome bd complex
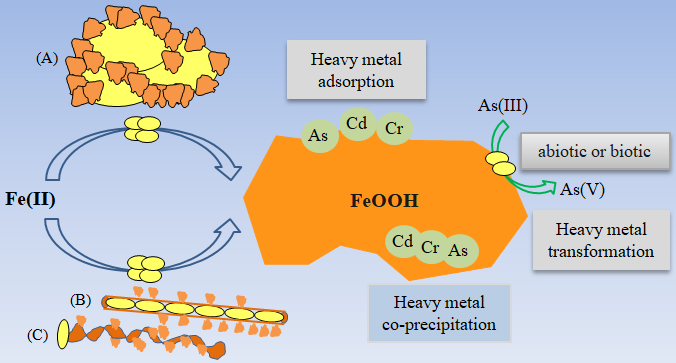
图3 微氧生物亚铁氧化成矿过程对重金属的转化及固定作用 (A)为结壳作用,(B)和(C)为形成的鞘状或螺旋杆状结构
Figure 3 Heavy metals transformation and immobilization of Fe(II) mineralization processes driven by microaerophilic Fe(II)-oxidizing bacteria
| [1] |
AMSTAETTER K, BORCH T, LARESE-CASANOVA P, et al., 2010. Redox transformation of arsenic by Fe(II)-activated goethite (α-FeOOH)[J]. Environmental Science & Technology, 44(1): 102-108.
DOI URL |
| [2] |
ANDREWS S C, ROBINSON A K, RODRÍGUEZ-QUIÑONES F, 2003. Bacterial iron homeostasis[J]. FEMS Microbiology Reviews, 27(2-3): 215-237.
PMID |
| [3] |
ARMSTRONG W, 1971. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging[J]. Physiologia Plantarum, 25(2): 192-197.
DOI URL |
| [4] |
BENZINE J, SHELOBOLINA E, XIONG M Y, et al., 2013. Fe-phyllosilicate redox cycling organisms from a redox transition zone in Hanford 300 Area sediments[J]. Frontiers in Microbiology, 4: 388.
DOI PMID |
| [5] | BLACKWELL N, BRYCE C, STRAUB D, et al., 2020. Genomic insights into two novel Fe(II)-oxidizing Zetaproteobacteria isolates reveal lifestyle adaption to coastal marine sediments[J]. Applied and Environmental Microbiology, 86(17): e01160-01120. |
| [6] |
BLÖTHE M, RODEN E E, 2009. Microbial iron redox cycling in a circumneutral-pH groundwater seep[J]. Applied and Environmental Microbiology, 75(2): 468-473.
DOI PMID |
| [7] |
CHAN C S, EMERSON D, LUTHER G W III, 2016. The role of microaerophilic Fe-oxidizing microorganisms in producing banded iron formations[J]. Geobiology, 14(5): 509-528.
DOI URL |
| [8] |
CHAN C S, FAKRA S C, EMERSON D, et al., 2011. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation[J]. The ISME Journal, 5: 717-727.
DOI URL |
| [9] |
CHEN Y, LI X, LIU T, et al., 2022. Metagenomic analysis of Fe(II)-oxidizing bacteria for Fe(III) mineral formation and carbon assimilation under microoxic conditions in paddy soil[J]. Science of the Total Environment, 851(1): 158068.
DOI URL |
| [10] |
CROSBY H A, JOHNSON C M, RODEN E E, et al., 2005. Coupled Fe(II)-Fe(III) electron and atom exchange as a mechanism for Fe isotope fractionation during dissimilatory iron oxide reduction[J]. Environmental Science & Technology, 39(17): 6698-6704.
DOI URL |
| [11] |
DANGETI S, MCBETH J M, ROSHANI B, et al., 2020. Microbial communities and biogenic Mn-oxides in an on-site biofiltration system for cold Fe(II)- and Mn(II)-rich groundwater treatment[J]. Science of the Total Environment, 710: 136386.
DOI URL |
| [12] | DEMIR N M, ATCI E B, DEMIR S, 2016. Effects of varying inlet iron and manganese concentrations on slow sand filter performance[J]. Sigma Journal of Engineering and Natural Sciences, 34(4): 505-515. |
| [13] |
DIXIT S, HERING J G, 2003. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implication for arsenic mobility[J]. Environmental Science & Technology, 37(18): 4182-4189.
DOI URL |
| [14] |
DONG L H, CHEN M J, LIU C S, et al., 2024. Microbe interactions drive the formation of floating iron films in circumneutral wetlands[J]. Science of the Total Environment, 906: 167711.
DOI URL |
| [15] |
DRUSCHEL G K, EMERSON D, SUTKA R, et al., 2008. Low-oxygen and chemical kinetic constraints on the geochemical niche of neutrophilic iron(II) oxidizing microorganisms[J]. Geochimica et Cosmochimica Acta, 72(14): 3358-3370.
DOI URL |
| [16] | EHRENBERG C G, 1837. Remarks on the real occurrence of fossils infusoria and their extensive diffusion[J]. Taylor's Scientific Memoirs, 1: 400-413. |
| [17] |
EMERSON D, 2009. Potential for iron-reduction and iron-cycling in iron oxyhydroxide-rich microbial mats at Loihi Seamount[J]. Geomicrobiology Journal, 26(8): 639-647.
DOI URL |
| [18] | EMERSON D, DE VET W, 2015. The role of FeOB in engineered water ecosystems: A review[J]. Journal-American Water Works Association, 107(1): E47-E57. |
| [19] |
EMERSON D, FIELD E K, CHERTKOV O, et al., 2013. Comparative genomics of freshwater Fe-oxidizing bacteria: Implications for physiology, ecology, and systematics[J]. Frontiers in Microbiology, 4: 254.
DOI PMID |
| [20] |
EMERSON D, FLEMING E J, MCBETH J M, 2010. Iron-oxidizing bacteria: An environmental and genomic perspective[J]. Annual Review of Microbiology, 64(1): 561-583.
DOI URL |
| [21] |
EMERSON D, FLOYD M M, 2005. Enrichment and isolation of iron-oxidizing bacteria at neutral pH[J]. Methods in Enzymology, 397: 112-123.
PMID |
| [22] |
EMERSON D, MOYER C, 1997. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH[J]. Applied and Environmental Microbiology, 63(12): 4784-4792.
DOI PMID |
| [23] |
EMERSON D, REVSBECH N P, 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: field studies[J]. Applied and Environmental Microbiology, 60(11): 4022-4031.
DOI PMID |
| [24] |
FERNANDEZ-ROJO L, HÉRY M, LE PAPE P, et al., 2017. Biological attenuation of arsenic and iron in a continuous flow bioreactor treating acid mine drainage (AMD)[J]. Water Research, 123: 594-606.
DOI URL |
| [25] |
FLEMING E J, CETINIĆ I, CHAN C S, et al., 2014. Ecological succession among iron-oxidizing bacteria[J]. The ISME Journal, 8: 804-815.
DOI URL |
| [26] |
FLEMING E J, LANGDON A E, MARTINEZ-GARCIA M, et al., 2011. What's new is old: resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and FISH[J]. PlOS ONE, 6(3): e17769.
DOI URL |
| [27] |
FREDRICKSON J K, ZACHARA J M, KUKKADAPU R K, et al., 2001. Biotransformation of Ni-substituted hydrous ferric oxide by an Fe(III)- reducing bacterium[J]. Environmental Science & Technology, 35(4): 703-712.
DOI URL |
| [28] | GARRISON C E, PRICE K A, FIELD E K, 2019. Environmental evidence for and genomic insight into the preference of iron-oxidizing bacteria for more-corrosion-resistant stainless steel at higher salinities[J]. Applied and Environmental Microbiology, 85(14): e00483-00419. |
| [29] |
GLAZER B T, ROUXEL O J, 2009. Redox speciation and distribution within diverse iron-dominated microbial habitats at Loihi Seamount[J]. Geomicrobiology Journal, 26(8): 606-622.
DOI URL |
| [30] |
GÜLAY A, ÇEKIÇ Y, MUSOVIC S, et al., 2018. Diversity of iron oxidizers in groundwater-fed rapid sand filters: evidence of Fe(II)-dependent growth by Curvibacter and Undibacterium spp[J]. Frontiers in Microbiology, 9: 2808.
DOI URL |
| [31] | HALLBECK L, STHL F, PEDERSEN K, 1993. Phytogeny and phenotypic characterization of the stalk-forming and iron-oxidizing bacterium Gallionella ferruginea[J]. Microbiology, 139(7): 1531-1535. |
| [32] | HANERT H, 1968. Untersuchungen zur Isolierung, Stoffwechselphysiologie und Morphologie von Gallionella ferruginea Ehrenberg[J]. Archives of Microbiology, 60: 348-376. |
| [33] | HASSAN Z, SULTANA M, VAN BREUKELEN B M, et al., 2015. Diverse arsenic-and iron-cycling microbial communities in arsenic- contaminated aquifers used for drinking water in Bangladesh[J]. FEMS Microbiology Ecology, 91(4): fiv026. |
| [34] |
HE S, BARCO R A, EMERSON D, et al., 2017. Comparative genomic analysis of neutrophilic iron(II) oxidizer genomes for candidate genes in extracellular electron transfer[J]. Frontiers in Microbiology, 8: 1584.
DOI PMID |
| [35] |
HUA J, FEI Y H, FENG C H, et al., 2022. Anoxic oxidation of As(III) during Fe(II)-induced goethite recrystallization: Evidence and importance of Fe(IV) intermediate[J]. Journal of Hazardous Materials, 421: 126806.
DOI URL |
| [36] |
JAMES R E, FERRIS F G, 2004. Evidence for microbial-mediated iron oxidation at a neutrophilic groundwater spring[J]. Chemical Geology, 212(3): 301-311.
DOI URL |
| [37] |
KAPPLER A, JOHNSON C M, CROSBY H A, et al., 2010. Evidence for equilibrium iron isotope fractionation by nitrate-reducing iron (II)-oxidizing bacteria[J]. Geochimica et Cosmochimica Acta, 74(10): 2826-2842.
DOI URL |
| [38] |
KAPPLER A, STRAUB K L, 2005. Geomicrobiological cycling of iron[J]. Reviews in Mineralogy and Geochemistry, 59(1): 85-108.
DOI URL |
| [39] |
KARIMIAN N, JOHNSTON S G, BURTON E D, 2017. Antimony and arsenic behavior during Fe(II)-induced transformation of jarosite[J]. Environmental Science & Technology, 51(8): 4259-4268.
DOI URL |
| [40] |
KATO S, CHAN C, ITOH T, et al., 2013. Functional gene analysis of freshwater iron-rich flocs at circumneutral pH and isolation of a stalk-forming microaerophilic iron-oxidizing bacterium[J]. Applied and Environmental Microbiology, 79(17): 5283-5290.
DOI PMID |
| [41] | KATO S, ITOH T, IINO T, et al., 2022. Sideroxyarcus emersonii gen. nov. sp. nov., a neutrophilic, microaerobic iron-and thiosulfate-oxidizing bacterium isolated from iron-rich wetland sediment[J]. International Journal of Systematic and Evolutionary Microbiology, 72(4): 005347. |
| [42] |
KATO S, OHKUMA M, POWELL D H, et al., 2015. Comparative genomic insights into ecophysiology of neutrophilic, microaerophilic iron oxidizing bacteria[J]. Frontiers in Microbiology, 6: 1265.
DOI PMID |
| [43] |
KATSOYIANNIS I A, ZOUBOULIS A I, 2004. Application of biological processes for the removal of arsenic from groundwaters[J]. Water Research, 38(1): 17-26.
PMID |
| [44] |
KATSOYIANNIS I A, ZOUBOULIS A I, 2006. Use of iron-and manganese-oxidizing bacteria for the combined removal of iron, manganese and arsenic from contaminated groundwater[J]. Water Quality Research Journal, 41(2): 117-129.
DOI URL |
| [45] |
KHALIFA A, NAKASUJI Y, SAKA N, et al., 2018. Ferrigenium kumadai gen. nov., sp. nov., a microaerophilic iron-oxidizing bacterium isolated from a paddy field soil[J]. International Journal of Systematic and Evolutionary Microbiology, 68(8): 2587-2592.
DOI URL |
| [46] |
KREPSKI S T, EMERSON D, HREDZAK-SHOWALTER P L, et al., 2013. Morphology of biogenic iron oxides records microbial physiology and environmental conditions: toward interpreting iron microfossils[J]. Geobiology, 11(5): 457-471.
DOI PMID |
| [47] |
KREPSKI S T, HANSON T E, CHAN C S, 2012. Isolation and characterization of a novel biomineral stalk-forming iron-oxidizing bacterium from a circumneutral groundwater seep[J]. Environmental Microbiology, 14(7): 1671-1680.
DOI PMID |
| [48] |
KUCERA S, WOLFE R, 1957. A selective enrichment method for Gallionella ferruginea[J]. Journal of Bacteriology, 74(3): 344-349.
DOI PMID |
| [49] | LAUFER K, NORDHOFF M, HALAMA M, et al., 2017. Microaerophilic Fe(II)-oxidizing Zetaproteobacteria isolated from low-Fe marine coastal sediments: Physiology and composition of their twisted stalks[J]. Applied and Environmental Microbiology, 83(8): e03118-16. |
| [50] |
LUO X H, JIANG X X, XUE S G, et al., 2021. Arsenic biomineralization by iron oxidizing strain (Ochrobactrum sp.) isolated from a paddy soil in Hunan, China[J]. Land Degradation & Development, 32(6): 2082-2093.
DOI URL |
| [51] | MAISCH M, LUEDER U, KAPPLER A, et al., 2019b. Iron lung: how rice roots induce iron redox changes in the rhizosphere and create niches for microaerophilic Fe(II)-oxidizing bacteria[J]. Environmental Science & Technology Letters, 6(10): 600-605. |
| [52] |
MAISCH M, LUEDER U, LAUFER K, et al., 2019a. Contribution of microaerophilic iron(II)-oxidizers to iron (III) mineral formation[J]. Environmental Science & Technology, 53(14): 8197-8204.
DOI URL |
| [53] |
MAKITA H, TANAKA E, MITSUNOBU S, et al., 2017. Mariprofundus micogutta sp. nov., a novel iron-oxidizing Zetaproteobacterium isolated from a deep-sea hydrothermal field at the Bayonnaise knoll of the Izu-Ogasawara arc, and a description of Mariprofundales ord. nov. and Zetaproteobacteria classis nov[J]. Archives of Microbiology, 199: 335-346.
DOI URL |
| [54] | MCALLISTER S M, MOORE R M, GARTMAN A, et al., 2019. The Fe(II)-oxidizing Zetaproteobacteria: historical, ecological and genomic perspectives[J]. FEMS Microbiology Ecology, 95(4): fiz015. |
| [55] |
MCBETH J M, LITTLE B J, RAY R I, et al., 2011. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments[J]. Applied and Environmental Microbiology, 77(4): 1405-1412.
DOI URL |
| [56] |
MITSUNOBU S, HAMANURA N, KATAOKA T, et al., 2013. Arsenic attenuation in geothermal streamwater coupled with biogenic arsenic(III) oxidation[J]. Applied Geochemistry, 35: 154-160.
DOI URL |
| [57] |
MUEHE E M, KAPPLER A, 2014. Arsenic mobility and toxicity in South and South-east Asia-a review on biogeochemistry, health and socio-economic effects, remediation and risk predictions[J]. Environmental Chemistry, 11: 483-495.
DOI URL |
| [58] | MÜHLING M, POEHLEIN A, STUHR A, et al., 2016. Reconstruction of the metabolic potential of acidophilic Sideroxydans strains from the metagenome of an microaerophilic enrichment culture of acidophilic iron-oxidizing bacteria from a pilot plant for the treatment of acid mine drainage reveals metabolic versatility and adaptation to life at low pH[J]. Frontiers in Microbiology, 7: 2082. |
| [59] |
NAKAGAWA K, MURASE J, ASAKAWA S, et al., 2020. Involvement of microaerophilic iron-oxidizing bacteria in the iron-oxidizing process at the surface layer of flooded paddy field soil[J]. Journal of Soils and Sediments, 20: 4034-4041.
DOI |
| [60] |
NARUSE T, BAN Y, YOSHIDA T, et al., 2019. Community structure of microaerophilic iron-oxidizing bacteria in Japanese paddy field soils[J]. Soil Science and Plant Nutrition, 65(5): 460-470.
DOI URL |
| [61] |
NEUBAUER S C, EMERSON D, MEGONIGAL J P, 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere[J]. Applied and Environmental Microbiology, 68(8): 3988-3995.
DOI PMID |
| [62] |
NITZSCHE K S, LAN V M, TRANG P T K, et al., 2015a. Arsenic removal from drinking water by a household sand filter in Vietnam-Effect of filter usage practices on arsenic removal efficiency and microbiological water quality[J]. Science of The Total Environment, 502: 526-536.
DOI URL |
| [63] |
NITZSCHE K S, WEIGOLD P, LÖSEKANN-BEHRENS T, et al., 2015b. Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam[J]. Chemosphere, 138: 47-59.
DOI URL |
| [64] |
NOTINI L, LATTA D E, NEUMANN, et al., 2018. The role of defects in Fe(II)-goethite electron transfer[J]. Environmental Science & Technology, 52: 2751-2759.
DOI URL |
| [65] |
PICARDAL F W, ZAYBAK Z, CHAKRABORTY A, et al., 2011. Microaerophilic, Fe(II)-dependent growth and Fe (II) oxidation by a Dechlorospirillum species[J]. FEMS Microbiology Letters, 319(1): 51-57.
DOI URL |
| [66] |
QIAN Z Y, WU C, PAN W S, et al., 2022. Arsenic transformation in soil-rice system affected by iron-oxidizing strain (Ochrobactrum sp.) and related soil metabolomics analysis[J]. Frontiers in Microbiology, 13: 794950.
DOI URL |
| [67] |
RENTZ J A, KRAIYA C, LUTHER G W, et al., 2007. Control of ferrous iron oxidation within circumneutral microbial iron mats by cellular activity and autocatalysis[J]. Environmental Science & Technology, 41(17): 6084-6089.
DOI URL |
| [68] |
RODEN E E, SOBOLEV D, GLAZER B, et al., 2004. Potential for microscale bacterial Fe redox cycling at the aerobic-anaerobic interface[J]. Geomicrobiology Journal, 21(6): 379-391.
DOI URL |
| [69] | SHELOBOLINA E, KONISHI H, XU H, et al., 2012. Isolation of phyllosilicate-iron redox cycling microorganisms from an illite- smectite rich hydromorphic soil[J]. Frontiers in Microbiology, 3: 134. |
| [70] | SIERING P L, GHIORSE W C, 1996. Phylogeny of the Sphaerotilus- Leptothrix group inferred from morphological comparisons, genomic fingerprinting, and 16S ribosomal DNA sequence analyses[J]. International Journal of Systematic and Evolutionary Microbiology, 46(1): 173-182. |
| [71] |
SINGER E, EMERSON D, WEBB E A, et al., 2011. Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium[J]. PlOS ONE, 6(9): e25386.
DOI URL |
| [72] | SINGH V K, SINGH A L, SINGH R, et al., 2018. Iron oxidizing bacteria: insights on diversity, mechanism of iron oxidation and role in management of metal pollution[J]. Environmental Sustainability, 1: 221-231. |
| [73] |
SOBOLEV D, RODEN E E, 2001. Suboxic deposition of ferric iron by bacteria in opposing gradients of Fe(II) and oxygen at circumneutral pH[J]. Applied and Environmental Microbiology, 67(3): 1328-1334.
PMID |
| [74] |
SOWERS T D, HARRINGTON J M, POLIZZOTTO M L, et al., 2017. Sorption of arsenic to biogenic iron (oxyhydr) oxides produced in circumneutral environments[J]. Geochimica et Cosmochimica Acta, 198: 194-207.
DOI URL |
| [75] |
ST CLAIR B, POTTENGER J, DEBES R, et al., 2019. Distinguishing biotic and abiotic iron oxidation at low temperatures[J]. ACS Earth and Space Chemistry, 3(6): 905-921.
DOI |
| [76] |
STRAUB K L, BENZ M, SCHINK B, et al., 1996. Anaerobic, nitrate- dependent microbial oxidation of ferrous iron[J]. Applied and Environmental Microbiology, 62(4): 1458-1460.
DOI URL |
| [77] |
STRAUB K L, SCHÖNHUBER W A, BUCHHOLZ-CLEVEN B E, et al., 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling[J]. Geomicrobiology Journal, 21(6): 371-378.
DOI URL |
| [78] | STUMM W, MORGAN J, 1996. Aquatic chemistry: Chemical equilibria and rates in natural waters[J]. Journal of Chemical Education, 73(11): A277. |
| [79] | SUMMERS Z M, GRALNICK J A, BOND D R, 2013. Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes[J]. mBio, 4(1): 10-1128. |
| [80] |
SWANNER E D, WU W, SCHOENBERG R, et al., 2015. Fractionation of Fe isotopes during Fe(II) oxidation by a marine photoferrotroph is controlled by the formation of organic Fe-complexes and colloidal Fe fractions[J]. Geochimica et Cosmochimica Acta, 165: 44-61.
DOI URL |
| [81] |
TIAN Z Y, FENG Y, GUAN Y Y, et al., 2017. Opposite effects of dissolved oxygen on the removal of As(III) and As(V) by carbonate structural Fe(II)[J]. Scientific Reports, 7: 17015.
DOI PMID |
| [82] |
TONG H, LIU C S, HAO L K, et al., 2019. Biological Fe(II) and As(III) oxidation immobilizes arsenic in micro-oxic environments[J]. Geochimica et Cosmochimica Acta, 265: 96-108.
DOI URL |
| [83] |
TONG H, ZHENG C J, LI B, et al., 2021. Microaerophilic oxidation of Fe(II) coupled with simultaneous carbon fixation and As(III) oxidation and sequestration in karstic paddy soil[J]. Environmental Science & Technology, 55(6): 3634-3644.
DOI URL |
| [84] |
WARD L M, IDEI A, TERAJIMA S, et al., 2017. Microbial diversity and iron oxidation at Okuoku-hachikurou Onsen, a Japanese hot spring analog of Precambrian iron formations[J]. Geobiology, 15(6): 817-835.
DOI PMID |
| [85] |
WEISS J V, RENTZ J A, PLAIA T, et al., 2007. Characterization of neutrophilic Fe(II)-oxidizing bacteria isolated from the rhizosphere of wetland plants and description of Ferritrophicum radicicola gen. nov. sp. nov., and Sideroxydans paludicola sp. nov[J]. Geomicrobiology Journal, 24(7-8): 559-570.
DOI URL |
| [86] |
WU K K, WU C, JIANG X X, et al., 2022. Remediation of arsenic-contaminated paddy field by a new iron oxidizing strain (Ochrobactrum sp.) and iron-modified biochar[J]. Journal of Environmental Sciences, 115: 411-421.
DOI URL |
| [87] |
YU H Y, WANG X Q, LI F B, et al., 2017. Arsenic mobility and bioavailability in paddy soil under iron compound amendments at different growth stages of rice[J]. Environmental Pollution, 224: 136-147.
DOI URL |
| [88] |
ZHAO Z W, MENG Y, YUAN Q K, et al., 2021. Microbial mobilization of arsenic from iron-bearing clay mineral through iron, arsenate, and simultaneous iron-arsenate reduction pathways[J]. Science of the Total Environment, 763: 144613.
DOI URL |
| [89] | ZHOU N Q, KEFFER J L, POLSON S W, et al., 2022b. Unraveling Fe(II)-oxidizing mechanisms in a facultative Fe(II) oxidizer, Sideroxydans lithotrophicus strain ES-1, via culturing, transcriptomics, and reverse transcription-quantitative PCR[J]. Applied and Environmental Microbiology, 88(2): e01595-01521. |
| [90] |
ZHOU N, KUPPER R J, CATALANO J G, et al., 2022a. Biological oxidation of Fe(II)-bearing smectite by microaerophilic iron oxidizer Sideroxydans lithotrophicus using dual Mto and Cyc2 iron oxidation pathways[J]. Environmental Science & Technology, 56(23): 17443-17453.
DOI URL |
| [91] | 林超峰, 龚骏, 2012. 嗜中性微好氧铁氧化菌研究进展[J]. 生态学报, 32(18): 5889-5899. |
|
LIN C F, GONG J, 2012. Recent progress in research on neutrophilic, microaerophilic iron(II)-oxidizing bacteria[J]. Acta Ecologica Sinica, 32(18): 5889-5899.
DOI URL |
|
| [92] | 刘亚楠, 陈曼佳, 童辉, 等, 2018. 亚铁驱动针铁矿晶相重组耦合砷氧化机制[J]. 矿物学报, 39(5): 572-579. |
| LIU Y N, CHEN M J, TONG H, et al., 2018. Oxidation mechanism of Fe(II)aq-induced crystalline phase reconstruction of goethite coupling with As(III)[J]. Acta mineralogical Sinica, 39(5): 572-579. |
| [1] | 江润海, 温绍福, 朱城强, 张梅, 杨润玲, 王春雪, 侯秀丽. 铅污染矿区中耐铅解磷菌对玉米的促生及根际铅的固化效应[J]. 生态环境学报, 2024, 33(2): 291-300. |
| [2] | 杨正桥, 邹奇, 韦行, 周凯, 陈志良. 金属尾矿微生物对尾矿环境的适应与调控机制研究进展[J]. 生态环境学报, 2024, 33(1): 156-166. |
| [3] | 刘炳妤, 王一佩, 姚作芳, 杨钙仁, 徐晓楠, 邓羽松, 黄钰涵. 沼液还田下不同种植模式的重金属风险评价及安全消纳量分析[J]. 生态环境学报, 2023, 32(8): 1507-1515. |
| [4] | 王宁, 刘效东, 甘先华, 苏宇乔, 吴国章, 黄芳芳, 张卫强. 亚热带典型林分降水过程中的水质效应[J]. 生态环境学报, 2023, 32(8): 1365-1375. |
| [5] | 杜丹丹, 高瑞忠, 房丽晶, 谢龙梅. 旱区盐湖盆地土壤重金属空间变异及对土壤理化因子的响应[J]. 生态环境学报, 2023, 32(6): 1123-1132. |
| [6] | 冯树娜, 吕家珑, 何海龙. KI淋洗对黄绵土汞污染的去除效果及土壤理化性状的影响[J]. 生态环境学报, 2023, 32(4): 776-783. |
| [7] | 陈敏毅, 朱航海, 佘伟铎, 尹光彩, 黄祖照, 杨巧玲. 珠三角某遗留造船厂场地土壤重金属人体健康风险评估及源解析[J]. 生态环境学报, 2023, 32(4): 794-804. |
| [8] | 陈敏毅, 宋清梅, 叶权运, 游学睿, 吴颖欣. 华南典型金属制品遗留生产场地重金属空间分布特征[J]. 生态环境学报, 2023, 32(12): 2228-2235. |
| [9] | 肖洁芸, 周伟, 石佩琪. 土壤重金属含量高光谱反演[J]. 生态环境学报, 2023, 32(1): 175-182. |
| [10] | 马闯, 王雨阳, 周通, 吴龙华. 污染土壤颗粒态有机质镉锌富集特征及其解吸行为研究[J]. 生态环境学报, 2022, 31(9): 1892-1900. |
| [11] | 黄宏, 郑欣芸, 李迎东, 赵旭, 俞锦辰, 汪振华. 大陈岛海域不同年龄褐菖鲉对重金属富集作用研究[J]. 生态环境学报, 2022, 31(9): 1885-1891. |
| [12] | 李莹, 张洲, 杨高明, 祖艳群, 李博, 陈建军. 湿地植物根系泌氧能力和根表铁膜与根系吸收重金属的关系[J]. 生态环境学报, 2022, 31(8): 1657-1666. |
| [13] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| [14] | 罗松英, 李秋霞, 邱锦坤, 邓素炎, 李一锋, 陈碧珊. 南三岛土壤-红树植物系统中重金属形态特征及迁移转化规律[J]. 生态环境学报, 2022, 31(7): 1409-1416. |
| [15] | 董乐恒, 王旭刚, 陈曼佳, 王子豪, 孙丽蓉, 石兆勇, 吴琪琪. 光照和避光条件下石灰性水稻土Fe氧化还原与Cu活性关系研究[J]. 生态环境学报, 2022, 31(7): 1448-1455. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
