生态环境学报 ›› 2025, Vol. 34 ›› Issue (4): 621-630.DOI: 10.16258/j.cnki.1674-5906.2025.04.011
吴昕优1,2( ), 涂晨1,2, 刘国明1, 杨帅1, 王译1,2, 王旭洋1,3, 骆润来4,5, 李忠元4,5, 骆永明1,2,*(
), 涂晨1,2, 刘国明1, 杨帅1, 王译1,2, 王旭洋1,3, 骆润来4,5, 李忠元4,5, 骆永明1,2,*( )
)
收稿日期:2025-01-06
出版日期:2025-04-18
发布日期:2025-04-24
通讯作者:
*骆永明。E-mail: ymluo@iaaas.ac.cn作者简介:吴昕优(1999年生),女,硕士研究生,研究方向为土壤重金属污染与减量修复。E-mail: xinyouwu1211@163.com
基金资助:
WU Xinyou1,2( ), TU Chen1,2, LIU Guoming1, YANG Shuai1, WANG Yi1,2, WANG Xuyang1,3, LUO Runlai4,5, LI Zhongyuan4,5, LUO Yongming1,2,*(
), TU Chen1,2, LIU Guoming1, YANG Shuai1, WANG Yi1,2, WANG Xuyang1,3, LUO Runlai4,5, LI Zhongyuan4,5, LUO Yongming1,2,*( )
)
Received:2025-01-06
Online:2025-04-18
Published:2025-04-24
摘要: 面对严峻的环境镉(Cd)污染问题,磁性吸附材料作为一种高效吸附Cd且环境友好的修复材料,受到广泛关注。前期以四氧化三铁(Fe3O4)为磁性基体,通过与巯基改性黏土矿物凹凸棒复合,制备了毫米级磁性复合黏土矿物修复材料,并成功用于土壤中Cd的吸附去除,实现了土壤减污修复。然而,目前对毫米级磁性复合黏土矿物修复材料的结构性质缺乏系统表征,其对Cd的吸附特征尚不明确,吸附机理亦缺少探讨。基于此,通过多种材料分析手段系统表征了毫米级磁性复合黏土矿物修复材料的微观特征、结构性质,结合吸附动力学与等温吸附模型探讨了其对Cd的吸附特征,探究了材料结构与功能的相互关系,分析了其对Cd的吸附机理。结果表明,毫米级磁性复合黏土矿物修复材料呈球形(直径为1.27 mm),表面褶皱多孔,具有尺寸适宜、富含官能团、结构稳定性强、可磁性回收的独特优势,对Cd的吸附过程符合准二级动力学模型和Langmuir等温模型,最大理论吸附量为46.14 mg·g−1。此外,毫米级磁性复合黏土矿物修复材料对Cd的吸附可能涉及多种作用机制,包括孔隙内扩、配位络合、离子交换、静电吸附作用等。该研究可为认识毫米级磁性复合黏土矿物修复材料的特征与减污净土作用提供科学依据。
中图分类号:
吴昕优, 涂晨, 刘国明, 杨帅, 王译, 王旭洋, 骆润来, 李忠元, 骆永明. 毫米级磁性复合黏土矿物修复材料的结构、性质及其对镉的吸附特征[J]. 生态环境学报, 2025, 34(4): 621-630.
WU Xinyou, TU Chen, LIU Guoming, YANG Shuai, WANG Yi, WANG Xuyang, LUO Runlai, LI Zhongyuan, LUO Yongming. Structural, Physicochemical and Cadmium Adsorption Properties of Millimeter-Scale Magnetic Composite Clay-Based Remediation Materials[J]. Ecology and Environment, 2025, 34(4): 621-630.
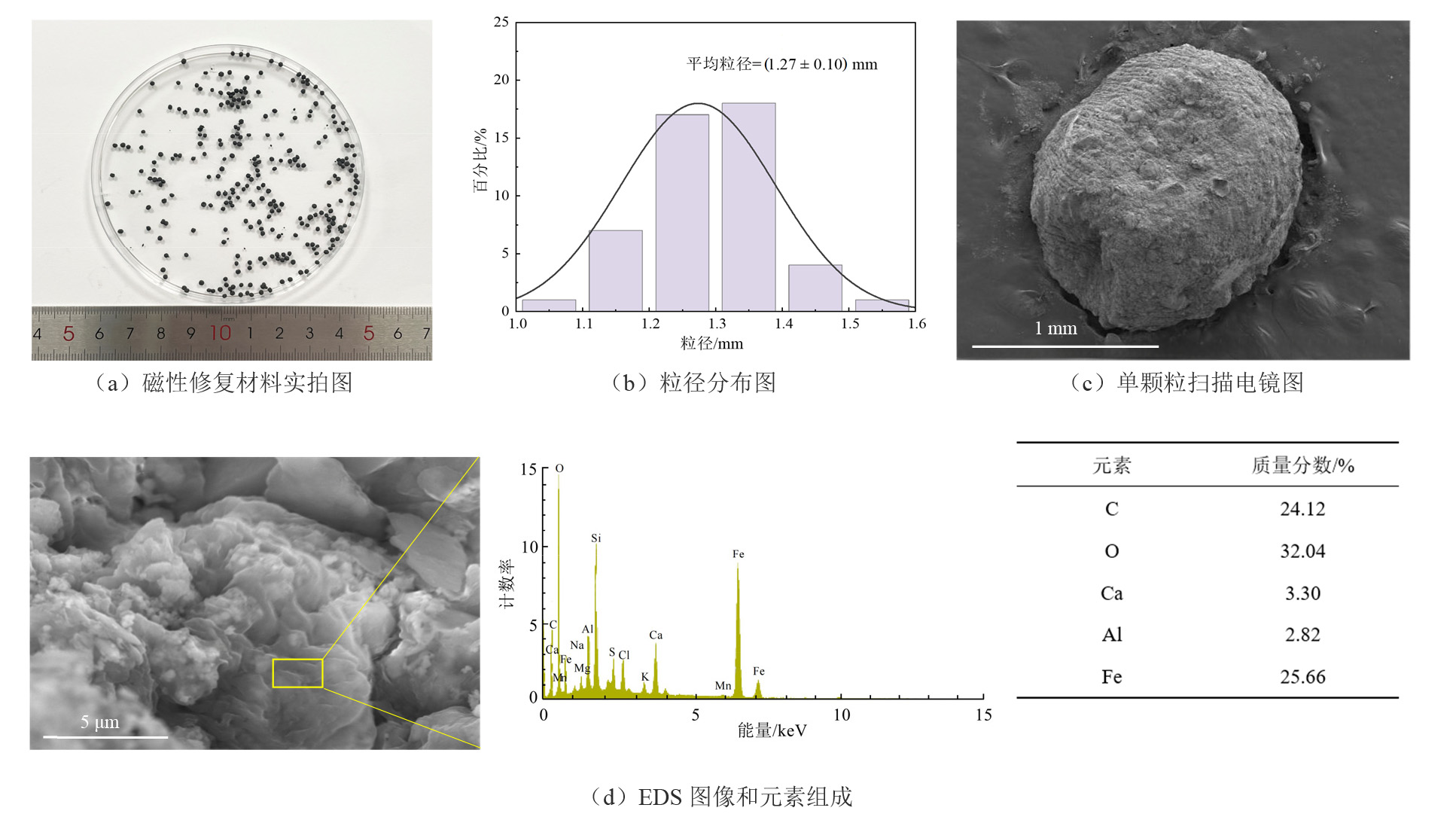
图1 毫米级磁性复合黏土矿物修复材料的扫描电镜图(SEM)
Figure 1 Scanning Electron Microscope (SEM) images of millimeter-scale magnetic composite clay-based remediation materials
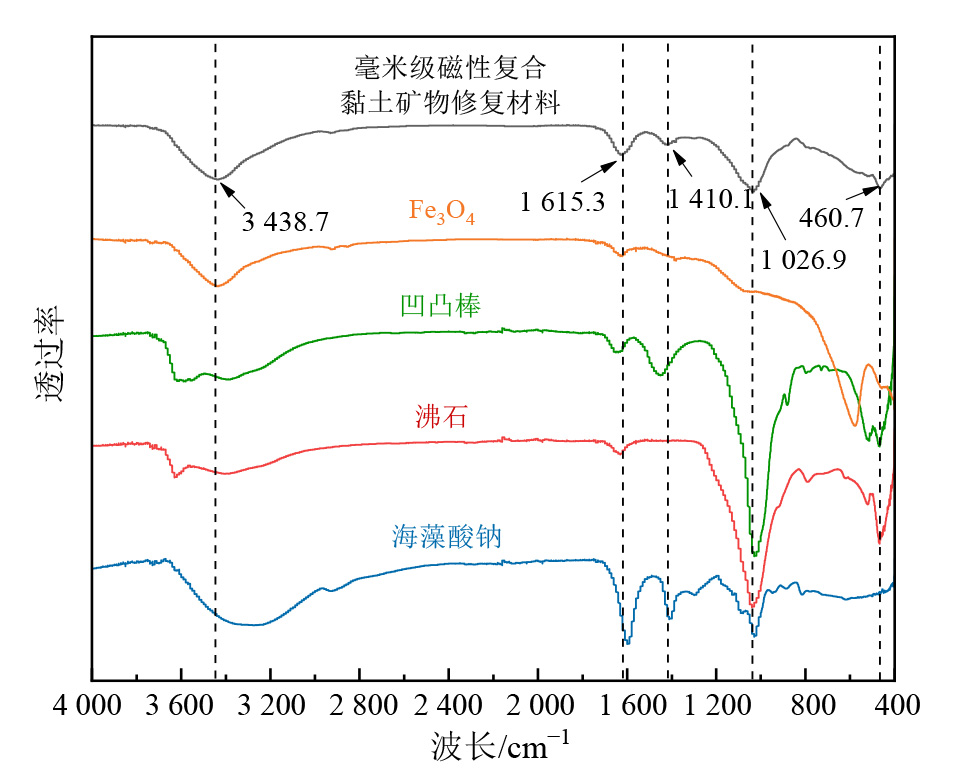
图3 毫米级磁性复合黏土矿物修复材料及其原始组分的FTIR光谱图
Figure 3 The FTIR spectrum of millimeter-scale magnetic composite clay-based remediation materials and its raw constituents
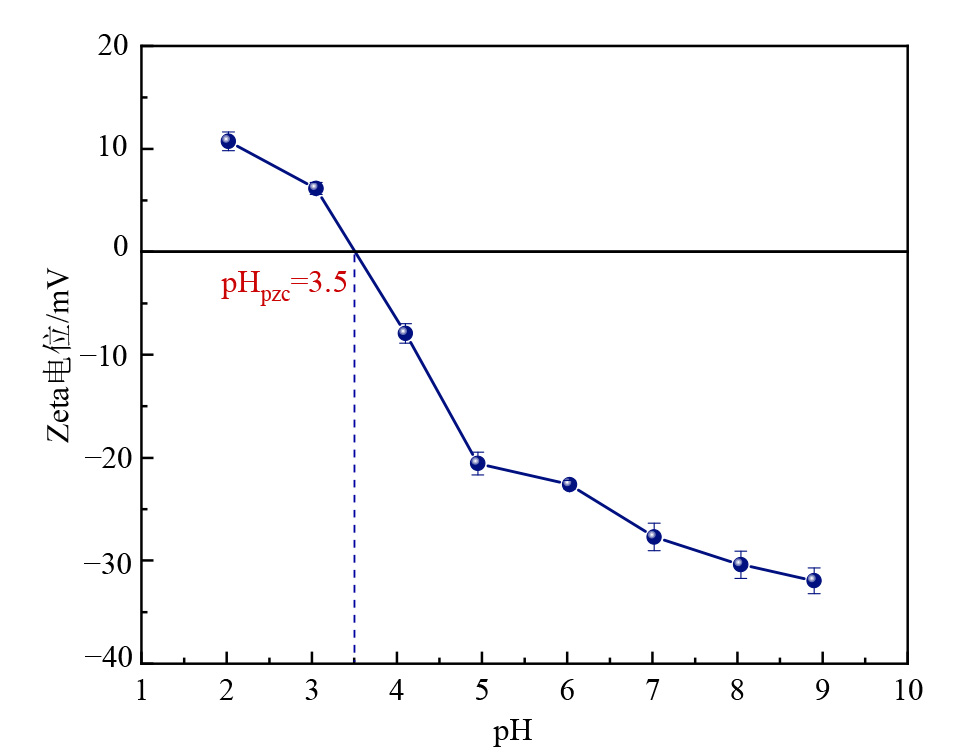
图5 毫米级磁性复合黏土矿物修复材料的Zeta电位与pH值的关系
Figure 5 Relationship between Zeta potential and pH value of millimeter-scale magnetic composite clay-based remediation materials
| 准一级动力学拟合 | 准二级动力学拟合 | Elovich模型拟合 | 颗粒内扩散模型 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg·g−1) | k1 | R2 | Qe/(mg·g−1) | k2 | R2 | α | β | R2 | ki1 | R | ki2 | R2 | |||
| 26.06 | 0.01 | 0.989 | 31.39 | 1.34 | 0.997 | 0.19 | 0.13 | 0.971 | 1.15 | 0.990 | 0.24 | 0.983 | |||
表1 毫米级磁性复合黏土矿物修复材料对Cd的吸附动力学拟合参数
Table 1 Fitting parameters of the adsorption kinetics of Cd on millimeter-scale magnetic composite clay-based remediation materials
| 准一级动力学拟合 | 准二级动力学拟合 | Elovich模型拟合 | 颗粒内扩散模型 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg·g−1) | k1 | R2 | Qe/(mg·g−1) | k2 | R2 | α | β | R2 | ki1 | R | ki2 | R2 | |||
| 26.06 | 0.01 | 0.989 | 31.39 | 1.34 | 0.997 | 0.19 | 0.13 | 0.971 | 1.15 | 0.990 | 0.24 | 0.983 | |||
| 吸附等温线 模型拟合 | Langmuir拟合 | Freundlich拟合 | |||||
|---|---|---|---|---|---|---|---|
| qmax/(mg·g−1) | kL | R2 | kF | 1/n | R2 | ||
| 46.14 | 0.054 | 0.961 | 8.85 | 0.328 | 0.947 | ||
表2 毫米级磁性复合黏土矿物修复材料对Cd的吸附等温线拟合参数
Table 2 Fitting parameters of the adsorption isotherm of Cd on millimeter-scale magnetic composite clay-based remediation materials
| 吸附等温线 模型拟合 | Langmuir拟合 | Freundlich拟合 | |||||
|---|---|---|---|---|---|---|---|
| qmax/(mg·g−1) | kL | R2 | kF | 1/n | R2 | ||
| 46.14 | 0.054 | 0.961 | 8.85 | 0.328 | 0.947 | ||
| [1] | BULIN C, ZHENG R X, SONG J L, et al., 2023. Magnetic graphene oxide-chitosan nanohybrid for efficient removal of aqueous Hg(Ⅱ) and the interaction mechanism[J]. Journal of Molecular Liquids, 370: 121050. |
| [2] | CHEN R P, ZHANG Y L, SHEN L F, et al., 2015. Lead(Ⅱ) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid[J]. Chemical Engineering Journal, 268: 348-355. |
| [3] | CUI M K, JIAO H T, YUAN S J, et al., 2024. Develop reusable carbon sub-micrometer composites with record-high Cd(Ⅱ) removal capacity[J]. Advanced Science, 12(3): 2570014. |
| [4] | FACCHI D P, CAZETTA A L, CANESIN E A, et al., 2018. New magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites applied for removal of Pb(Ⅱ) ions from aqueous systems[J]. Chemical Engineering Journal, 337: 595-608. |
| [5] | GHAFIL A J, MAZLOOM G, ABDI J, et al., 2024. Ti3C2Tx/ZIF-67 hybrid nanocomposite as a highly effective adsorbent for Pb(Ⅱ) removal from water: Synthesis and DFT calculations[J]. Applied Surface Science, 643: 158642. |
| [6] | GOUMA V, TZIASIOU C, POURNARA A D, et al., 2022. A novel approach to sorbent-based remediation of soil impacted by organic micropollutants and heavy metals using granular biochar amendment and magnetic separation[J]. Journal of Environmental Chemical Engineering, 10(2): 107316. |
| [7] | HOSSEINI S S, HAMADI A, FOROUTAN R, et al., 2022. Decontamination of Cd2+ and Pb2+ from aqueous solution using a magnetic nanocomposite of eggshell/starch/Fe3O4[J]. Journal of Water Process Engineering, 48: 102911. |
| [8] | JI J M, XIE W L, 2021. Removal of aflatoxin B1 from contaminated peanut oils using magnetic attapulgite[J]. Food Chemistry, 339: 128072. |
| [9] | KHOSHRAFTAR Z, MASOUMI H, GHAEMI A, 2023. On the performance of perlite as a mineral adsorbent for heavy metals ions and dye removal from industrial wastewater: A review of the state of the art[J]. Case Studies in Chemical and Environmental Engineering, 8: 100385. |
| [10] |
LEI T, JIANG X, ZHOU Y, et al., 2023. A multifunctional adsorbent based on 2,3-dimercaptosuccinic acid/dopamine-modified magnetic iron oxide nanoparticles for the removal of heavy-metal ions[J]. Journal of Colloid and Interface Science, 636: 153-166.
DOI PMID |
| [11] | LIANG X F, XU Y M, TAN X, et al., 2013. Heavy metal adsorbents mercapto and amino functionalized palygorskite: preparation and characterization[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 426: 98-105. |
| [12] | LIU G M, TU C, LI Y, et al., 2024. Rapidly reducing cadmium from contaminated farmland soil by novel magnetic recyclable Fe3O4/ mercapto-functionalized attapulgite beads[J]. Environmental Pollution, 351: 124056. |
| [13] | LIU K, QIN Y L, MUHAMMAD Y, et al., 2019. Effect of Fe3O4 content and microwave reaction time on the properties of Fe3O4/ZnO magnetic nanoparticles[J]. Journal of Alloys and Compounds, 781: 790-799. |
| [14] | LU L, SIM J, ZHAO R R, 2024. Mechanics of hard-magnetic soft materials: A review[J]. Mechanics of Materials, 189: 104874. |
| [15] | MA B, WANG J Y, ZHANG L, 2023. Two cadmium-resistant strains of agricultural soil effective in remediating soil cadmium pollution[J]. Journal of Environmental Chemical Engineering, 11(6): 111189. |
| [16] | MA J, MA Y, YU F, et al., 2018. Rotating magnetic field-assisted adsorption mechanism of pollutants on mechanically strong sodium alginate/graphene/l-cysteine beads in batch and fixed-bed column systems[J]. Environmental Science & Technology, 52(23): 13925-13934. |
| [17] | MESDAGHINIA A, AZARI A, NODEH R N, et al., 2017. Removal of phthalate esters (PAEs) by zeolite/Fe3O4: investigation on the magnetic adsorption separation, catalytic degradation and toxicity bioassay[J]. Journal of Molecular Liquids, 233: 378-390. |
| [18] | MO Z L, TAI D Z, ZHANG H, et al., 2022. A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water[J]. Chemical Engineering Journal, 443: 136320. |
| [19] | NG N T, KEYON A S A, IBRAHIM W A W, et al., 2023. Amino-functionalised chrysin as adsorbent in dispersive micro-solid phase extraction of selected heavy metal ions from stingless bee honey[J]. Journal of Food Composition and Analysis, 123: 105561. |
| [20] | OTUNOLA B O, OLOLAD O O, 2020. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes[J]. Environmental Technology & Innovation, 18: 100692. |
| [21] | PATHAK P D, MANDAVGANE S A, 2015. Preparation and characterization of raw and carbon from banana peel by microwave activation: Application in citric acid adsorption[J]. Journal of Environmental Chemical Engineering, 3(4): 2435-2447. |
| [22] | QIAN Y, FU P X, YIN R Z, et al., 2024. Preparation of bifunctional electrocatalyst by recycling heavy metal ions from wastewater using EDTAD-functionalized MOF as highly efficient adsorbent[J]. Rare Metals, 43(10): 5105-5116. |
| [23] |
RAI P K, LEE S S, ZHANG M, et al., 2019. Heavy metals in food crops: Health risks, fate, mechanisms, and management[J]. Environment International, 125: 365-385.
DOI PMID |
| [24] | SALEM D B, OUAKOUAK A, TOUAHRA F, et al., 2023. Easy separable, floatable, and recyclable magnetic-biochar/alginate bead as super-adsorbent for adsorbing copper ions in water media[J]. Bioresource Technology, 383: 129225. |
| [25] | SING K S W, 1985. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 57(4): 603-619. |
| [26] | SUN J H, CHEN Y, YU H Q, et al., 2018. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide[J]. Journal of Colloid and Interface Science, 532: 474-484. |
| [27] | SUN P, ZHANG W, ZOU B Z, et al., 2021. Efficient adsorption of Cu(Ⅱ), Pb(Ⅱ) and Ni(Ⅱ) from waste water by PANI@APTS-magnetic attapulgite composites[J]. Applied Clay Science, 209: 106151. |
| [28] | WANG L L, SHI Y, YAO D K, et al., 2019. Cd complexation with mercapto-functionalized attapulgite (MATP): adsorption and DFT study[J]. Chemical Engineering Journal, 366: 569-576. |
| [29] | WANG S J, LIU X X, ZHANG C Y, et al., 2025. Adsorption and selective mechanism of Pb2+ and Cd2+ on the surface of calcined modified attapulgite[J]. Separation and Purification Technology, 353(Part C): 128377. |
| [30] |
WANG Y Q, FENG Y, ZHANG X F, et al., 2018. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals[J]. Journal of Colloid and Interface Science, 514: 190-198.
DOI PMID |
| [31] | WU S S, GUO J, WANG Y, et al., 2021. Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution[J]. Journal of Materials Science, 56: 13096-13107. |
| [32] | XIANG X W, MAO X Y, DING X Q, et al., 2024. Assembly of core-shell Fe3O4@CD-MOFs derived hollow magnetic microcubes for efficient extraction of hazardous substances: Plausible mechanisms for selective adsorption[J]. Journal of Hazardous Materials, 473: 134588. |
| [33] | XIE S J, HUANG L, SU C Q, et al., 2024. Application of clay minerals as adsorbents for removing heavy metals from the environment[J]. Green and Smart Mining Engineering, 1(3): 249-261. |
| [34] | XU C L, FENG Y L, LI H R, et al., 2022. Adsorption of heavy metal ions by iron tailings: Behavior, mechanism, evaluation and new perspectives[J]. Journal of Cleaner Production, 344: 131065. |
| [35] |
XU J W, LIU C, HSU P-C, et al., 2019. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry[J]. Nature Communications, 10(1): 2440.
DOI PMID |
| [36] | YANG R L, ZHANG Q, SHI J, et al., 2023. A novel magnetic loading porous liquid absorbent for removal of Cu(Ⅱ) and Pb(Ⅱ) from the aqueous solution[J]. Separation and Purification Technology, 314: 123605. |
| [37] | YANG S, LI Y, SHI S C et al., 2022. Feasibility of a combined solubilization and eluent drainage system to remove Cd and Cu from agricultural soil[J]. Science of the Total Environment, 807(Part 2): 150733. |
| [38] | ZHANG Y K, WU X G, T Y, et al., 2023. Effect of plant growth-promoting rhizobacteria on oilseed rape Brassica juncea and phytoextraction of cadmium[J]. Journal of Soils and Sediments, 23(9): 3472-3484. |
| [39] | ZHANG Y M, REN Y L, ZU Y, et al., 2024. Disassembly-reassembly-phosphating strategy to fabricate hydrothermally-stable hierarchical P@ZSM-5 zeolite for efficient methanol-to-propylene[J]. Chemical Engineering Journal, 497: 154755. |
| [40] | ZHAO H H, HUANG X R, ZHANG G B, et al., 2020. Possibility of removing cadmium pollution from the environment using a newly synthesized material coal fly ash[J]. Environmental Science and Pollution Research, 27: 4997-5008. |
| [41] | 胡志龙, 孙寒雪, 魏慧娟, 等, 2019. 多孔材料在去除废水中重金属离子方面的研究进展[J]. 化工新型材料, 47(7): 46-49. |
| HU Z L, SUN H X, WEI H J, et al., 2019. Research progress of porous material applied in removing heavy metal ions from wastewater[J]. New Chemical Materials, 47(7): 46-49. | |
| [42] | 骆永明, 滕应, 2020. 中国土壤污染与修复科技研究进展和展望[J]. 土壤学报, 57(5): 1137-1142. |
| LUO Y M, TENG Y, 2020. Research progress and prospects on soil pollution and remediation in China[J]. Acta Pedologica Sinica, 57(5): 1137-1142. | |
| [43] | 王启豪, 刘国明, 涂晨, 等, 2023. MgAl-LDHs磁性颗粒对镉污染农田土壤的减量修复研究[J]. 土壤, 55(6): 1297-1305. |
| WANG Q H, LIU G M, TU C, et al., 2023. Reduced remediation of cadmium contaminated farmland soil by Mg-Al-Layered double hydroxide magnetic particles[J]. Soils, 55(6): 1297-1305. | |
| [44] |
邢献军, 罗甜, 卜玉蒸, 等, 2023. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 42(3): 1527-1539.
DOI |
| XING X J, LOU T, BU Y Z, et al., 2023. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption[J]. Chemical Industry and Engineering Progress, 42(3): 1527-1539. | |
| [45] | 贠豪, 李远, 杨帅, 等, 2021. 磁性黏土颗粒对污染土壤中镉去除作用的初步研究[J]. 土壤, 53(5): 1015-1022. |
| YUN H, LI Y, YANG S, et al., 2021. Preliminary study on removal of cadmium from contaminated soils by magnetic clay particles[J]. Soils, 53(5): 1015-1022. | |
| [46] |
杨新衡, 纪志永, 郭志远, 等, 2024. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 43(9): 5262-5274.
DOI |
|
YANG X H, JI Z Y, GUO Z Y, et al., 2024. Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance[J]. Chemical Industry and Engineering Progress, 43(9): 5262-5274.
DOI |
| [1] | 崔雪丹, 段桂兰, 王向琴, 李志丰, 窦飞, 杜衍红, 袁雨珍, 刘传平, 李芳柏. 基于两地长期定位试验的铁改性木本泥炭修复中轻度镉砷污染稻田效果与土壤健康效应评价[J]. 生态环境学报, 2025, 34(4): 608-620. |
| [2] | 宁静, 王淳, 卢莞玲, 韦露. 斑马鱼暴露于镉和褪黑素引起肠道组织、氧化损伤及微生物多样性变化[J]. 生态环境学报, 2025, 34(1): 77-88. |
| [3] | 曹振宇, 涂晨, 刘颖, 韩军超, 邢倩雯, 骆永明. 趋磁细菌Magnetospirillum gryphiswaldense MSR-1对镉的生物吸附初步研究[J]. 生态环境学报, 2025, 34(1): 99-107. |
| [4] | 李林峰, 徐梓盛, 陈勇, 李奇, 林晓扬, 李义纯. 施硅水平对水稻根表铁膜和体内Cd累积分布的影响[J]. 生态环境学报, 2024, 33(5): 781-790. |
| [5] | 刘楚天, 郭栋栋, 侯磊, 梁启斌, 王艳霞, 施艳婷, 戚艳娥. 营养调控影响滇杨幼苗镉积累的效应模型分析[J]. 生态环境学报, 2024, 33(3): 460-468. |
| [6] | 张腾云, 王静, 高健磊, 葛文静, 王宗耀, 韩龙. 碱性农田土壤冬小麦不同生育期镉的迁移转化研究[J]. 生态环境学报, 2024, 33(3): 450-459. |
| [7] | 官国庆, 黄紫琳, 江龙飞, 罗春玲. 伴矿景天对重金属-多环芳烃复合污染土壤有机污染物消减及微生物的影响[J]. 生态环境学报, 2024, 33(12): 1931-1943. |
| [8] | 纪晟莹, 李杰, 李鑫, 陶禹, 陈娟, 王晓玉. 环境与基因型互作对瓜类蔬菜镉积累的影响及产地土壤安全阈值研究[J]. 生态环境学报, 2024, 33(12): 1944-1952. |
| [9] | 范婉仪, 涂晨, 王顺扬, 吴昕优, 李烜桢, 骆永明. 不同品种烟草对轻度污染耕地土壤中镉的累积特征与减量修复潜力[J]. 生态环境学报, 2023, 32(8): 1516-1524. |
| [10] | 李治梅, 安娅, 李梅, 王室苹, 秦好丽. 巯基/铁基功能化蒙脱土对土壤镉的钝化行为研究[J]. 生态环境学报, 2023, 32(7): 1301-1312. |
| [11] | 王丽华, 王磊, 许端平, 薛杨. 煤胶体对重金属铜与镉的吸附特征研究[J]. 生态环境学报, 2023, 32(7): 1293-1300. |
| [12] | 李振国, 郝星雨, 贺甜莲, 景蕊, 荣成, 顾承真, 郑新宇. 竹醋液对紫苏镉毒的缓解效应研究[J]. 生态环境学报, 2023, 32(7): 1313-1324. |
| [13] | 赵良侠, 高坤, 黄婷婷, 高也, 琚唐丹, 蒋秋阳, 金珩, 熊蕾, 汤在琳, 高灿红. 玉米籽粒高/低镉积累自交系不同生育期的镉累积特性研究[J]. 生态环境学报, 2023, 32(4): 766-775. |
| [14] | 杨耀东, 陈玉梅, 涂鹏飞, 曾清如. 经济作物轮作模式下镉污染农田修复潜力[J]. 生态环境学报, 2023, 32(3): 627-634. |
| [15] | 陈桂红. 硫和硅掺杂生物炭对镉污染土壤的修复研究[J]. 生态环境学报, 2023, 32(10): 1854-1860. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
