生态环境学报 ›› 2023, Vol. 32 ›› Issue (7): 1293-1300.DOI: 10.16258/j.cnki.1674-5906.2023.07.012
收稿日期:2023-03-08
出版日期:2023-07-18
发布日期:2023-09-27
通讯作者:
* 薛杨。E-mail: xueyangqq@126.com作者简介:王丽华(1966年生),女,副教授,研究方向为环境土壤修复与生态重建。E-mail: 157887697@qq.com
基金资助:
WANG Lihua( ), WANG Lei, XU Duanping, XUE Yang*(
), WANG Lei, XU Duanping, XUE Yang*( )
)
Received:2023-03-08
Online:2023-07-18
Published:2023-09-27
摘要:
煤矿周围含有大量的煤胶体,其比表面积大,对污染物质的吸附能力更强。煤胶体进入土壤后,可能对环境中的重金属迁移有促进作用,目前,相关文献较少。因此,研究煤胶体对重金属污染物的吸附特征,可以为预测重金属在煤矿区周围土壤中的归趋提供理论依据。采集辽宁省阜新市长焰煤,提取不同粒级的煤胶体(0-2、2-5、5-10 μm),并研究其对重金属污染物铜与镉的吸附动力学和热力学行为。结果表明:煤胶体粒径越大,其比表面积越小,有机质含量越少。准二级动力学方程能更好的描述各粒级煤胶体对铜与镉的吸附动力学过程(r2>0.99),表明煤胶体对铜与镉的吸附以化学吸附为主。铜镉在0-2、2-5、5-10 μm粒级煤胶体上的吸附速率常数分别为:0.0818、0.0796、0.0536,镉的分别为0.0717、0.0631、0.0336,表明煤胶体粒径越大,对铜与镉的吸附速率越小,且煤胶体对铜的吸附速率大于镉,铜达到吸附平衡的时间更短。铜在0-2、2-5、5-10 μm粒级煤胶体上的平衡吸附量分别为:19.3、18.4、14.3 mg·g-1,镉的分别为25.5、20.6、17.5 mg·g-1,表明煤胶体粒径越大,对铜与镉的平衡吸附量越小,且煤胶体对镉的平衡吸附量大于对铜的平衡吸附量。Freundlich吸附等温模型能更好的描述各粒级煤胶体对铜与镉的等温吸附过程,温度对吸附过程有显著影响,温度越高,煤胶体对铜与镉的吸附能力越强。此外,各粒级煤胶体对铜和镉的吸附热力学参数ΔG0<0、ΔH>0、ΔS>0,表明煤胶体对铜和镉的吸附是可自发的、熵增的吸热反应,为化学吸附,煤胶体粒径的减小也提高对铜与镉的吸附能力。
中图分类号:
王丽华, 王磊, 许端平, 薛杨. 煤胶体对重金属铜与镉的吸附特征研究[J]. 生态环境学报, 2023, 32(7): 1293-1300.
WANG Lihua, WANG Lei, XU Duanping, XUE Yang. Adsorption Characteristics of Copper and Cadmium on Coal Colloid[J]. Ecology and Environment, 2023, 32(7): 1293-1300.
| 粒级/ μm | BET比表面积/(m2·g-1) | 总孔容量/ (cm3·g-1) | 平均孔径/ nm | 所需粒径质量分数/% | 有机质质量分数/% |
|---|---|---|---|---|---|
| 0-2 | 50.922± 1.461 | 0.0830± 0.0174 | 108.977± 0.949 | 85.792± 1.041 | 17.523± 0.515 |
| 2-5 | 25.581± 0.994 | 0.0281± 0.0022 | 166.013± 0.586 | 75.037± 0.879 | 15.473± 0.945 |
| 5-10 | 11.384± 1.044 | 0.0173± 0.0061 | 170.273± 0.667 | 61.285± 0.863 | 10.785± 0.487 |
表1 煤胶体的基本理化性质
Table 1 Basic physical and chemical properties of coal colloid
| 粒级/ μm | BET比表面积/(m2·g-1) | 总孔容量/ (cm3·g-1) | 平均孔径/ nm | 所需粒径质量分数/% | 有机质质量分数/% |
|---|---|---|---|---|---|
| 0-2 | 50.922± 1.461 | 0.0830± 0.0174 | 108.977± 0.949 | 85.792± 1.041 | 17.523± 0.515 |
| 2-5 | 25.581± 0.994 | 0.0281± 0.0022 | 166.013± 0.586 | 75.037± 0.879 | 15.473± 0.945 |
| 5-10 | 11.384± 1.044 | 0.0173± 0.0061 | 170.273± 0.667 | 61.285± 0.863 | 10.785± 0.487 |
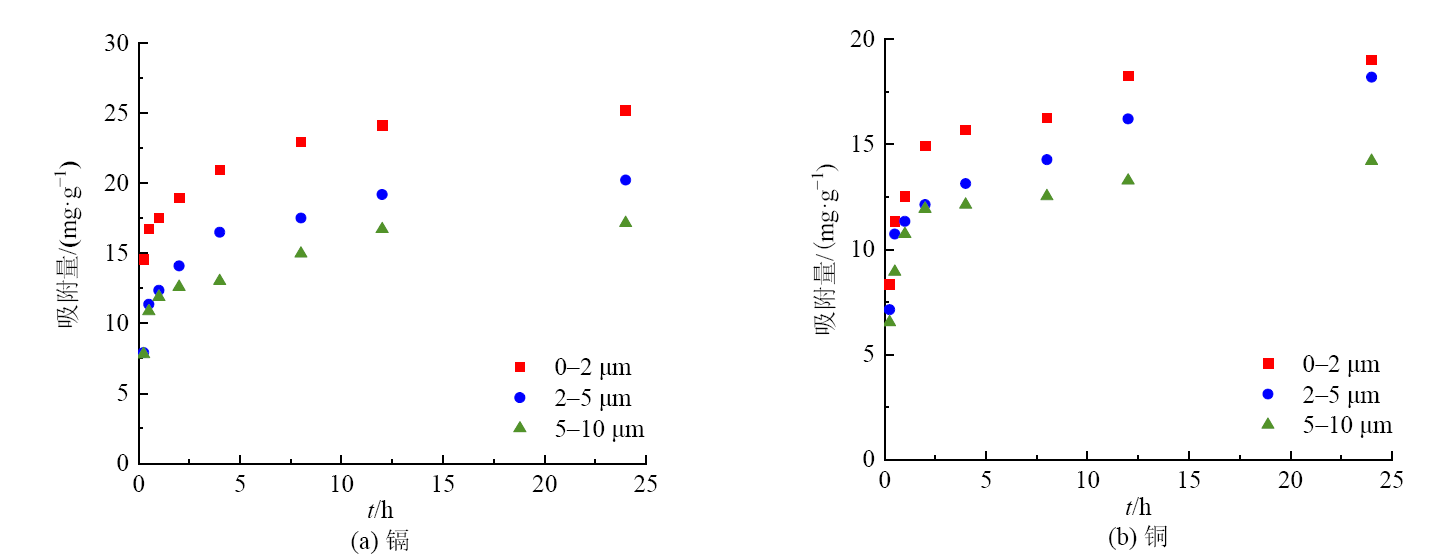
图2 煤胶体在不同粒级下(0-2、2-5、5-10 μm)对(a)镉与(b)铜的吸附动力学曲线
Figure 2 Adsorption kinetic curve of coal colloid ( 0-2, 2-5, 5-10 μm) for (a) cadmium and (b) copper under different particle sizes
| 重金属 | 粒级/ μm | 准一级 | 准二级 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | Qe | r2 | Qe | K2 | t1/2 | r2 | |||
| 铜 | 0-2 | 2.102 | 16.707 | 0.799 | 19.292 | 0.0818 | 0.0328 | 0.998 | |
| 2-5 | 2.262 | 14.685 | 0.664 | 18.379 | 0.0796 | 0.0372 | 0.992 | ||
| 5-10 | 2.449 | 12.738 | 0.893 | 14.294 | 0.0536 | 0.0913 | 0.998 | ||
| 镉 | 0-2 | 3.388 | 21.829 | 0.552 | 25.537 | 0.0717 | 0.0214 | 0.999 | |
| 2-5 | 1.707 | 17.585 | 0.774 | 20.610 | 0.0631 | 0.0373 | 0.998 | ||
| 5-10 | 2.528 | 14.735 | 0.696 | 17.490 | 0.0336 | 0.0973 | 0.997 | ||
表2 不同粒级煤胶体对铜与镉吸附动力学方程拟合结果
Table 2 Fitting results of adsorption kinetic equation of copper and cadmium on coal colloids of different particle sizes
| 重金属 | 粒级/ μm | 准一级 | 准二级 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | Qe | r2 | Qe | K2 | t1/2 | r2 | |||
| 铜 | 0-2 | 2.102 | 16.707 | 0.799 | 19.292 | 0.0818 | 0.0328 | 0.998 | |
| 2-5 | 2.262 | 14.685 | 0.664 | 18.379 | 0.0796 | 0.0372 | 0.992 | ||
| 5-10 | 2.449 | 12.738 | 0.893 | 14.294 | 0.0536 | 0.0913 | 0.998 | ||
| 镉 | 0-2 | 3.388 | 21.829 | 0.552 | 25.537 | 0.0717 | 0.0214 | 0.999 | |
| 2-5 | 1.707 | 17.585 | 0.774 | 20.610 | 0.0631 | 0.0373 | 0.998 | ||
| 5-10 | 2.528 | 14.735 | 0.696 | 17.490 | 0.0336 | 0.0973 | 0.997 | ||
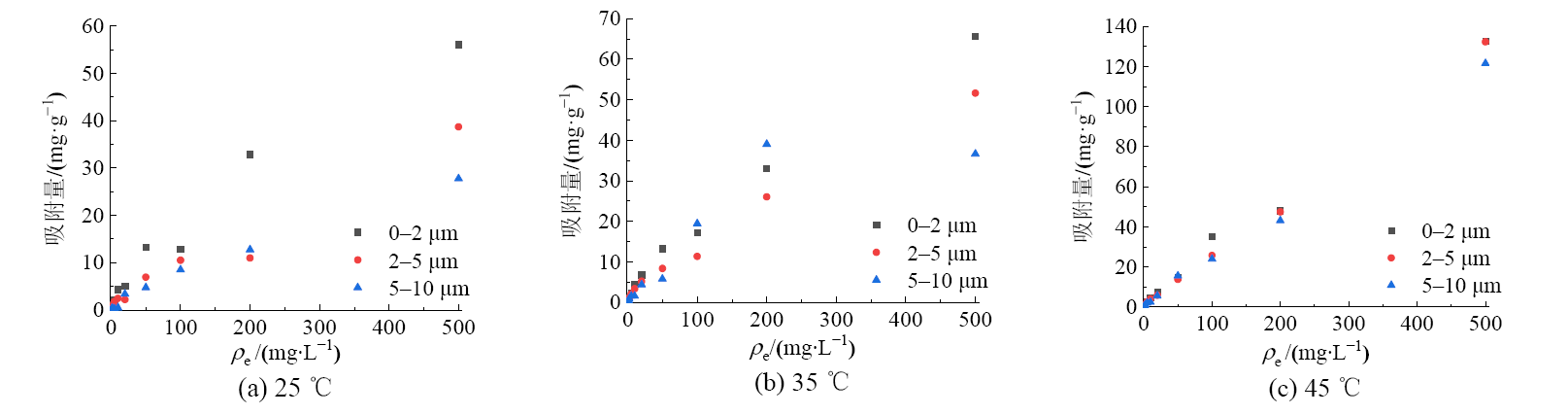
图4 0-2、2-5、5-10 μm煤胶体在不同温度下对镉的等温吸附曲线
Figure 4 Isothermal adsorption curves of 0-2, 2-5 and 5-10 μm coal colloids for cadmium at different temperatures
| 重金属 | 粒级/ μm | 温度/ ℃ | Linear | Langmuir | Frendlich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kc | r2 | a | KL | r2 | n | KF | r2 | |||||
| 铜 | 0-2 | 25 | 0.0598 | 0.974 | 21.275 | 0.0112 | 0.774 | 0.781 | 1.093 | 0.965 | ||
| 35 | 0.0559 | 0.940 | 26.920 | 0.0087 | 0.872 | 0.572 | 1.260 | 0.946 | ||||
| 45 | 0.0441 | 0.960 | 31.437 | 0.0040 | 0.970 | 0.469 | 1.784 | 0.970 | ||||
| 2-5 | 25 | 0.0725 | 0.728 | 22.434 | 0.0275 | 0.932 | 0.465 | 1.174 | 0.938 | |||
| 35 | 0.0625 | 0.975 | 30.328 | 0.0266 | 0.980 | 0.464 | 1.611 | 0.996 | ||||
| 45 | 0.0441 | 0.613 | 33.676 | 0.0178 | 0.955 | 0.326 | 1.803 | 0.921 | ||||
| 5-10 | 25 | 0.0828 | 0.959 | 24.671 | 0.0074 | 0.822 | 0.552 | 2.018 | 0.999 | |||
| 35 | 0.0655 | 0.936 | 30.762 | 0.0098 | 0.884 | 0.533 | 2.066 | 0.956 | ||||
| 45 | 0.0469 | 0.962 | 37.871 | 0.0117 | 0.945 | 0.498 | 2.396 | 0.923 | ||||
| 镉 | 0-2 | 25 | 0.1343 | 0.937 | 26.165 | 0.0100 | 0.807 | 0.617 | 1.016 | 0.963 | ||
| 35 | 0.0884 | 0.964 | 39.668 | 0.0064 | 0.842 | 1.258 | 1.237 | 0.970 | ||||
| 45 | 0.0552 | 0.917 | 56.136 | 0.0093 | 0.895 | 0.502 | 1.361 | 0.908 | ||||
| 2-5 | 25 | 0.1648 | 0.980 | 37.120 | 0.0026 | 0.938 | 0.698 | 1.158 | 0.992 | |||
| 35 | 0.1164 | 0.996 | 51.601 | 0.0056 | 0.956 | 0.788 | 1.288 | 0.997 | ||||
| 45 | 0.0813 | 0.878 | 65.599 | 0.0024 | 0.910 | 0.542 | 1.373 | 0.965 | ||||
| 5-10 | 25 | 0.5208 | 0.983 | 130.582 | 0.0094 | 0.858 | 1.229 | 1.196 | 0.994 | |||
| 35 | 0.4574 | 0.977 | 132.627 | 0.0072 | 0.813 | 1.238 | 1.798 | 0.988 | ||||
| 45 | 0.4582 | 0.992 | 140.501 | 0.0075 | 0.810 | 1.125 | 1.954 | 0.994 | ||||
表3 煤胶体在不同温度下动力学方程拟合结果
Table 3 Fitting results of coal colloid kinetic equation at different temperatures
| 重金属 | 粒级/ μm | 温度/ ℃ | Linear | Langmuir | Frendlich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kc | r2 | a | KL | r2 | n | KF | r2 | |||||
| 铜 | 0-2 | 25 | 0.0598 | 0.974 | 21.275 | 0.0112 | 0.774 | 0.781 | 1.093 | 0.965 | ||
| 35 | 0.0559 | 0.940 | 26.920 | 0.0087 | 0.872 | 0.572 | 1.260 | 0.946 | ||||
| 45 | 0.0441 | 0.960 | 31.437 | 0.0040 | 0.970 | 0.469 | 1.784 | 0.970 | ||||
| 2-5 | 25 | 0.0725 | 0.728 | 22.434 | 0.0275 | 0.932 | 0.465 | 1.174 | 0.938 | |||
| 35 | 0.0625 | 0.975 | 30.328 | 0.0266 | 0.980 | 0.464 | 1.611 | 0.996 | ||||
| 45 | 0.0441 | 0.613 | 33.676 | 0.0178 | 0.955 | 0.326 | 1.803 | 0.921 | ||||
| 5-10 | 25 | 0.0828 | 0.959 | 24.671 | 0.0074 | 0.822 | 0.552 | 2.018 | 0.999 | |||
| 35 | 0.0655 | 0.936 | 30.762 | 0.0098 | 0.884 | 0.533 | 2.066 | 0.956 | ||||
| 45 | 0.0469 | 0.962 | 37.871 | 0.0117 | 0.945 | 0.498 | 2.396 | 0.923 | ||||
| 镉 | 0-2 | 25 | 0.1343 | 0.937 | 26.165 | 0.0100 | 0.807 | 0.617 | 1.016 | 0.963 | ||
| 35 | 0.0884 | 0.964 | 39.668 | 0.0064 | 0.842 | 1.258 | 1.237 | 0.970 | ||||
| 45 | 0.0552 | 0.917 | 56.136 | 0.0093 | 0.895 | 0.502 | 1.361 | 0.908 | ||||
| 2-5 | 25 | 0.1648 | 0.980 | 37.120 | 0.0026 | 0.938 | 0.698 | 1.158 | 0.992 | |||
| 35 | 0.1164 | 0.996 | 51.601 | 0.0056 | 0.956 | 0.788 | 1.288 | 0.997 | ||||
| 45 | 0.0813 | 0.878 | 65.599 | 0.0024 | 0.910 | 0.542 | 1.373 | 0.965 | ||||
| 5-10 | 25 | 0.5208 | 0.983 | 130.582 | 0.0094 | 0.858 | 1.229 | 1.196 | 0.994 | |||
| 35 | 0.4574 | 0.977 | 132.627 | 0.0072 | 0.813 | 1.238 | 1.798 | 0.988 | ||||
| 45 | 0.4582 | 0.992 | 140.501 | 0.0075 | 0.810 | 1.125 | 1.954 | 0.994 | ||||
| 元素 | 粒级/ μm | t/ ℃ | K | ΔG0/ (KJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔH/ (KJ·mol-1) |
|---|---|---|---|---|---|---|
| 铜 | 0-2 | 25 | 1.10 | -0.211 | 8071 | 5.95 |
| 35 | 1.26 | -0.598 | ||||
| 45 | 1.78 | -1.525 | ||||
| 2-5 | 25 | 1.18 | -0.391 | 8007 | 5.84 | |
| 35 | 1.61 | -1.226 | ||||
| 45 | 1.80 | -1.550 | ||||
| 5-10 | 25 | 2.02 | -1.732 | 6772 | 3.78 | |
| 35 | 2.06 | -1.850 | ||||
| 45 | 2.40 | -2.307 | ||||
| 镉 | 0-2 | 25 | 1.01 | -0.031 | 8288 | 7.18 |
| 35 | 1.23 | -0.532 | ||||
| 45 | 1.37 | -0.818 | ||||
| 2-5 | 25 | 1.15 | -0.355 | 8153 | 6.65 | |
| 35 | 1.28 | -0.637 | ||||
| 45 | 1.37 | -0.831 | ||||
| 5-10 | 25 | 1.20 | -0.432 | 7949 | 5.58 | |
| 35 | 1.09 | -0.212 | ||||
| 45 | 1.26 | -0.598 |
表4 煤胶体吸附铜与镉的热力学参数
Table 4 Thermodynamic parameters of copper and cadmium adsorption by coal colloid
| 元素 | 粒级/ μm | t/ ℃ | K | ΔG0/ (KJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔH/ (KJ·mol-1) |
|---|---|---|---|---|---|---|
| 铜 | 0-2 | 25 | 1.10 | -0.211 | 8071 | 5.95 |
| 35 | 1.26 | -0.598 | ||||
| 45 | 1.78 | -1.525 | ||||
| 2-5 | 25 | 1.18 | -0.391 | 8007 | 5.84 | |
| 35 | 1.61 | -1.226 | ||||
| 45 | 1.80 | -1.550 | ||||
| 5-10 | 25 | 2.02 | -1.732 | 6772 | 3.78 | |
| 35 | 2.06 | -1.850 | ||||
| 45 | 2.40 | -2.307 | ||||
| 镉 | 0-2 | 25 | 1.01 | -0.031 | 8288 | 7.18 |
| 35 | 1.23 | -0.532 | ||||
| 45 | 1.37 | -0.818 | ||||
| 2-5 | 25 | 1.15 | -0.355 | 8153 | 6.65 | |
| 35 | 1.28 | -0.637 | ||||
| 45 | 1.37 | -0.831 | ||||
| 5-10 | 25 | 1.20 | -0.432 | 7949 | 5.58 | |
| 35 | 1.09 | -0.212 | ||||
| 45 | 1.26 | -0.598 |
| 范德华力 | 疏水作用 | 氢键 | 电荷转移 | 离子和配位基交换 | 偶极键 | 化学键 |
|---|---|---|---|---|---|---|
| 4.2-8.4 | ≈5 | 2-40 | - | ≈40 | 2-29 | 63-84 |
表5 不同作用引起的吸附反应焓变(薛杨,2017b)
Table 5 Enthalpy change of adsorption reaction caused by different actions kJ·moL-1
| 范德华力 | 疏水作用 | 氢键 | 电荷转移 | 离子和配位基交换 | 偶极键 | 化学键 |
|---|---|---|---|---|---|---|
| 4.2-8.4 | ≈5 | 2-40 | - | ≈40 | 2-29 | 63-84 |
| [1] |
BHATTACHARYYA P, CHAKRABARTI K, CHAKRABORTY A, et al., 2008. Fractionation and bioavailability of Pb in municipal solid waste compost and Pb uptake by rice straw and grain under submerged condition in amended soil[J]. Geosciences Journal, 12(1): 41-45.
DOI URL |
| [2] |
WAYCHUNAS G A, KIM C S, BANFIELD J F, 2005. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms[J]. Journal of Nanoparticle Research, 7(4-5): 409-433.
DOI URL |
| [3] |
GUALA S D, VEGA F A, COVELO E F, 2010. The dynamics of heavy metals in plant-soil interactions[J]. Ecological Modelling, 221(8): 1148-1152.
DOI URL |
| [4] |
JAMES S C, CHRYSIKOPOULOS C V, 1999. Transport of polydisperse colloid suspensions in a single fracture[J]. Water Resources Research, 35(3): 707-718.
DOI URL |
| [5] |
LIU F, XU B L, HE Y, et al., 2018. Differences in transport behavior of natural soil colloids of contrasting sizes from nanometer to micron and the environmental implications[J]. Science of the Total Environment, 634: 802-810
DOI URL |
| [6] |
LIU Y T, XU Z, HU X, et al., 2019. Sorption of Pb(II) and Cu(II) on the colloid of black soil, red soil and fine powder kaolinite: effects of pH, ionic strength and organic matter[J]. Environmental Pollutants and Bioavailability, 31(1): 85-93.
DOI URL |
| [7] |
MALIK P K, 2003. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of Acid Yellow 36[J]. Dyes and Pigments, 56(3): 239-249.
DOI URL |
| [8] |
MOLNAR I L, JOHNSON W P, GERHARD, et al., 2015. Predicting colloid transport through saturated porous media: A critical review[J]. Water Resources Research, 51(9): 6804-6845.
DOI URL |
| [9] | NUNEZ A G, 2010. Colloidal coal in water suspensions[J]. Energy & Environmental Science, 3(5): 629. |
| [10] |
ROSS P D, SUBRAMANIAN S, 1981. Thermodynamics of protein association reactions: forces contributing to stability[J]. Biochemistry, 20(11): 3096-3102.
DOI PMID |
| [11] |
SHEIN E V, DEVIN B A, 2007. Current problems in the study of colloidal transport in soil[J]. Eurasian Soil Science, 40(4): 399-408.
DOI URL |
| [12] |
SYNGOUNA V I, CHRYSIKOPOULOS C V, 2013. Cotransport of clay colloids and viruses in water saturated porous media[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 416: 56-65.
DOI URL |
| [13] |
TAN B, LIU C, TAN X, et al., 2022. Heavy metal transport driven by seawater-freshwater interface dynamics: The role of colloid mobilization and aquifer pore structure change[J]. Water Research, 217: 118370.
DOI URL |
| [14] |
WANG Y Z, LI H, LIN S H, 2022. Adsorption characteristics of modified bamboo charcoal on Cu(II) and Cd(II) in water[J]. Toxics, 10(12): 787.
DOI URL |
| [15] | 车轶夫, 2017. 典型重金属在黑土、胶体及水相中的分配特征[D]. 阜新: 辽宁工程技术大学. |
| CHE Y F, 2017. Distribution characteristics of typical heavy metals in black soil, colloids, and aqueous phases[D]. Fuxin:Liaoning Technical University. | |
| [16] | 陈付荣, 2022. 我国土壤重金属污染现状监测及其防治浅析[J]. 清洗世界, 38(8): 128-130. |
| CHEN F R, 2022. Monitoring and prevention of soil heavy metal pollution in China[J]. Cleaning World, 38(8): 128-130. | |
| [17] | 丁武泉, 何家洪, 刘新敏, 等, 2017. 有机质对三峡库区水体中土壤胶体颗粒凝聚影响机制研究[J]. 水土保持学报, 31(4): 166-171. |
| DING W Q, HE J H, LIU X M, et al., 2017. Study on the mechanism of the effect of organic matter on the coagulation of soil colloidal particles in the water body of the three gorges reservoir area[J]. Journal of Soil and Water Conservation, 31(4): 166-171. | |
| [18] | 窦红宾, 郭唯, 2022. 重金属污染及其对水土的危害[J]. 生态经济, 38(11): 5-8. |
| DOU H B, GUO W, 2022. Heavy metal pollution and its harm to soil and water[J]. Ecological Economy, 38(11): 5-8. | |
| [19] | 杜晓丽, 刘殿威, 崔申申, 2022. 径流入渗时土壤胶体释放对重金属截留的影响[J]. 中国环境科学, 42(3): 1278-1286. |
| DU X L, LIU D W, CUI S S, 2022. Impact of soil colloid release on heavy metal retention during runoff infiltration[J]. China Environmental Science, 42(3): 1278-1286. | |
| [20] | 葛华才, 查少秋, 万彩霞, 等, 2020. 液固吸附动力学与吸附等温式探索性实验[J]. 实验室研究与探索, 39(2): 5-8. |
| GE H C, CHA S Q, WAN C X, et al., 2020. Exploratory experiment on liquid-solid adsorption kinetics and adsorption isotherms[J]. Laboratory Research and Exploration, 39(2): 5-8. | |
| [21] |
龚仓, 马玲玲, 成杭新, 等, 2012. 典型农耕区黑土和沼泽土团聚体颗粒中重金属的分布特征解析[J]. 生态环境学报, 21(9): 1635-1639.
DOI |
| GONG C, MA L L, CHENG H X, et al., 2012. Characterization of the particle size fractionation associated heavy metals in typical black and bog arable soils[J]. Ecology and Environmental Sciences, 21(9): 1635-1639. | |
| [22] | 姜秀民, 李巨斌, 邱健荣, 2000. 超细化煤粉燃烧特性的研究[J]. 中国电机工程学报, 20(16): 71-74. |
| JIANG X M, LI J B, QIU J R, 2000. Research on the combustion characteristics of ultra-fine coal powder[J]. Proceedings of the CSEE, 20(16): 71-74. | |
| [23] | 李柏良, 2022. 不同离子强度/类型和pH对多孔介质中胶体协同Cu运移的影响研究[D]. 青岛: 青岛大学. |
| LI B L, 2022. Study on the effect of different ion strengths/types and pH on colloidal collaborative Cu migration in porous media[D]. Qingdao: Qingdao University. | |
| [24] | 李海燕, 2022. 土壤污染状况调查工作平台的设计与应用[J]. 低碳世界, 12(2): 37-39. |
| LI H Y, 2022. Design and application of a soil pollution investigation platform[J]. Low Carbon World, 12(2): 37-39. | |
| [25] | 林凡华, 陈海博, 白军, 2007. 土壤环境中重金属污染危害的研究[J]. 环境科学与管理, 32(7): 74-76. |
| LIN F H, CHEN H B, BAI J, 2007. Research on the hazards of heavy metal pollution in soil environment[J]. Environmental Science and Management, 32(7): 74-76. | |
| [26] | 刘霞, 2022. 重金属污染治理的环境保护优化策略探讨[J]. 山西化工, 42(2): 354-355. |
| LIU X, 2022. Exploration of environmental protection optimization strategies for heavy metal pollution control[J]. Shanxi Chemical Industry, 42(2): 354-355. | |
| [27] | 马义, 杨晋, 韩凤兰, 等, 2018. 脱硫石膏吸附水体中重金属离子行为的研究[J]. 硅酸盐通报, 37(6): 1868-1876, 1896. |
| MA Y, YANG J, HAN F L, et al., 2018. Study on the adsorption behavior of heavy metal ions in water by desulfurization gypsum[J]. Bulletin of the Chinese Ceramic Society, 37(6): 1868-1876, 1896. | |
| [28] | 牟海燕, 蒋茜茜, 吴晨伟, 等, 2019. 五种土壤胶体对重金属镉的吸附特征研究[J]. 四川大学学报(自然科学版), 56(6): 1125-1130. |
| MOU H Y, JIANG Q Q, WU C W, et al., 2019. Study on the adsorption characteristics of five soil colloids for heavy metal cadmium[J]. Journal of Sichuan University (Natural Science Edition), 56(6): 1125-1130. | |
| [29] | 中华人民共和国农业农村部, 2006. 土壤检测第6部分: 土壤有机质的测定: NY/T 1121.6-2006[S]. |
| Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2006. Soil testing Part 6: Determination of soil organic matter: NY/T 1121.6-2006[S]. | |
| [30] | 中华人民共和国环境保护部, 中华人民共和国国土资源部, 2014. 全国土壤污染状况调查公报[J]. 环境教育 (6): 7-10. |
| Ministry of Environmental Protection of the People’s Republic of China, Ministry of Land and Resources of the People’s Republic of China, 2014. National Soil Pollution Survey Bulletin[J]. Environmental Education (6): 8-10. | |
| [31] |
王玉洁, 刘蓓蓓, 万全, 等, 2021. 稀土元素在土壤中的释放与迁移研究进展[J]. 生态环境学报, 30(3): 644-654.
DOI |
| WANG Y J, LIU B B, WAN Q, et al., 2021. The study of the interaction of aqueous Fe(II) and lepidocrocite-humic acid compounds and the phase transformation[J]. Ecology and Environmental Sciences, 30(3): 644-654. | |
| [32] | 熊秋林, 赵佳茵, 赵文吉, 等, 2017. 北京市地表土重金属污染特征及潜在生态风险[J]. 中国环境科学, 37(6): 2211-2221. |
| XIONG Q L, ZHAO J Y, ZHAO W J, et al., 2017. Characteristics and potential ecological risks of heavy metal pollution in surface soil of Beijing[J]. China Environmental Science, 37(6): 2211-2221. | |
| [33] |
徐伟慧, 胡影, 王志刚. 2018. DEP和DBP在黑土胶体微界面的动力学行为[J]. 生态环境学报, 27(4): 752-760.
DOI |
| XU W H, HU Y, WANG Z G, 2018. Dynamic behavior of DEP and DBP developing on the interface of black soil colloid[J]. Ecology and Environmental Sciences, 27(4): 752-760. | |
| [34] | 许端平, 冯雨鑫, 王道涵, 等, 2014. 不同粒级黑土胶体对铅的等温吸附特征[J]. 环境工程学报, 8(11): 5015-5021. |
| XU D P, FENG Y X, WANG D H, et al., 2014. Isothermal adsorption characteristics of lead on black soil colloids of different particle sizes[J]. Journal of Environmental Engineering, 8(11): 5015-5021. | |
| [35] | 许信, 王龙瑛, 夏艳, 等, 2020. 土壤重金属污染的危害及修复技术研究[J]. 环境与发展, 32(5): 104-105. |
| XU X, WANG L Y, XIA Y, et al., 2020. Research on the hazards and remediation techniques of heavy metal pollution in soil[J]. Environment and Development, 32(5): 104-105. | |
| [36] | 薛杨, 邱素芬, 许端平, 等, 2017a. 不同粒级的煤胶体对汞的吸附动力学[J]. 环境工程学报, 11(5): 3187-3194. |
| XUE Y, QIU S F, XU D P, et al., 2017. Adsorption kinetics of mercury on coal colloids of different particle sizes[J]. Journal of Environmental Engineering, 11(5): 3187-3194. | |
| [37] | 薛杨, 邱素芬, 许端平, 等, 2017b. 不同粒级煤胶体对汞的吸附热力学[J]. 煤炭学报, 42(4): 1050-1055. |
| XUE Y, QIU S F, XU D P, et al., 2017. Thermodynamics of mercury adsorption by coal colloids of different particle sizes[J]. Journal of China Coal Society, 42(4): 1050-1055. | |
| [38] | 杨金燕, 杨肖娥, 何振立, 等, 2005. 土壤中铅的吸附-解吸行为研究进展[J]. 生态环境, 14(1):102-107. |
| YANG J Y, YANG X E, HE Z L, et al., 2005. Advance in the studies of Pb adsorption and desorption in soils[J]. Ecological Environment, 14(1): 102-107. | |
| [39] | 杨悦锁, 朱一丹, 张文卿, 等, 2020. 地下水系统中镍污染和天然胶体共迁移特征[J]. 吉林大学学报(地球科学版), 50(1): 226-233. |
| YANG Y S, ZHU Y D, ZHANG W Q, et al., 2020. Co migration characteristics of nickel pollution and natural colloids in groundwater systems[J]. Journal of Jilin University (Earth Science Edition), 50(1): 226-233. | |
| [40] | 张蓉蓉, 2020. 三种典型土壤胶体与耐性细菌对镉铅的吸附解吸特性研究[D]. 南宁: 广西大学. |
| ZHANG R R, 2020. Study on the adsorption and desorption characteristics of cadmium and lead by three typical soil colloids and tolerant bacteria[D]. Nanning: Guangxi University. | |
| [41] | 赵媛媛, 2018. 典型核素在红壤胶体的吸附性能研究[D]. 北京: 北京化工大学. |
| ZHAO Y Y, 2018. Study on the adsorption performance of typical nuclides on red soil colloids[D]. Beijing: Beijing University of Chemical Technology. | |
| [42] | 朱一丹, 2020. 多孔介质中土壤胶体与生物质炭对镉的吸附和迁移影响研究[D]. 长春: 吉林大学. |
| ZHU Y D, 2020. Study on the adsorption and migration of cadmium by soil colloids and biochar in porous media[D]. Changchun: Jilin University. |
| [1] | 李治梅, 安娅, 李梅, 王室苹, 秦好丽. 巯基/铁基功能化蒙脱土对土壤镉的钝化行为研究[J]. 生态环境学报, 2023, 32(7): 1301-1312. |
| [2] | 李振国, 郝星雨, 贺甜莲, 景蕊, 荣成, 顾承真, 郑新宇. 竹醋液对紫苏镉毒的缓解效应研究[J]. 生态环境学报, 2023, 32(7): 1313-1324. |
| [3] | 赵良侠, 高坤, 黄婷婷, 高也, 琚唐丹, 蒋秋阳, 金珩, 熊蕾, 汤在琳, 高灿红. 玉米籽粒高/低镉积累自交系不同生育期的镉累积特性研究[J]. 生态环境学报, 2023, 32(4): 766-775. |
| [4] | 杨宇, 邓仁健, 隆佩, 黄中杰, 任伯帜, 王政华. 砷氧化菌Pseudomonas sp. AO-1的分离鉴定及其对As(Ⅲ)的氧化性能研究[J]. 生态环境学报, 2023, 32(3): 619-626. |
| [5] | 杨耀东, 陈玉梅, 涂鹏飞, 曾清如. 经济作物轮作模式下镉污染农田修复潜力[J]. 生态环境学报, 2023, 32(3): 627-634. |
| [6] | 徐敏, 许超, 余光辉, 尹力初, 张泉, 朱捍华, 朱奇宏, 张杨珠, 黄道友. 地下水位和长期秸秆还田对土壤镉有效性及稻米镉含量的影响[J]. 生态环境学报, 2023, 32(1): 150-157. |
| [7] | 崔远远, 张征云, 刘鹏, 张运春, 张桥英. 镉与聚乙烯微塑料胁迫对小白菜根系的形态特征和分形维数的影响[J]. 生态环境学报, 2023, 32(1): 158-165. |
| [8] | 马闯, 王雨阳, 周通, 吴龙华. 污染土壤颗粒态有机质镉锌富集特征及其解吸行为研究[J]. 生态环境学报, 2022, 31(9): 1892-1900. |
| [9] | 李晓晖, 艾仙斌, 李亮, 王玺洋, 辛在军, 孙小艳. 新型改性稻壳生物炭材料对镉污染土壤钝化效果的研究[J]. 生态环境学报, 2022, 31(9): 1901-1908. |
| [10] | 李秀华, 赵玲, 滕应, 骆永明, 黄标, 刘冲, 刘本乐, 赵其国. 贵州汞矿区周边农田土壤汞镉复合污染特征空间分布及风险评估[J]. 生态环境学报, 2022, 31(8): 1629-1636. |
| [11] | 房献宝, 张智钧, 赖阳晴, 叶脉, 刁增辉. 新型污泥生物炭对土壤重金属Cr和Cd的修复研究[J]. 生态环境学报, 2022, 31(8): 1647-1656. |
| [12] | 龚玲玄, 王丽丽, 赵建宁, 刘红梅, 杨殿林, 张贵龙. 不同覆盖作物模式对茶园土壤理化性质及有机碳矿化的影响[J]. 生态环境学报, 2022, 31(6): 1141-1150. |
| [13] | 施建飞, 靳正忠, 周智彬, 王鑫. 额尔齐斯河流域典型尾矿库区周边土壤重金属污染评价[J]. 生态环境学报, 2022, 31(5): 1015-1023. |
| [14] | 赵超凡, 周丹丹, 孙建财, 钱坤鹏, 李芳芳. 生物炭中可溶性组分对其吸附镉的影响[J]. 生态环境学报, 2022, 31(4): 814-823. |
| [15] | 曾民, 陈佳, 李娥贤, 殷富有, 王玲仙, 曾黎琼, 郭蓉. 元江普通野生稻后代镉分布特点及镉积累动态变化规律[J]. 生态环境学报, 2022, 31(3): 565-571. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
