生态环境学报 ›› 2025, Vol. 34 ›› Issue (6): 853-862.DOI: 10.16258/j.cnki.1674-5906.2025.06.003
刘亚军1( ), 段亦鹏2, 李荣富1, 池泽涌1, 吴永明1,2,*(
), 段亦鹏2, 李荣富1, 池泽涌1, 吴永明1,2,*( )
)
收稿日期:2024-11-25
出版日期:2025-06-18
发布日期:2025-06-11
通讯作者:
* 吴永明, E-mail: 作者简介:刘亚军(1989年生),男,助理研究员,博士,主要从事环境微生物学研究。E-mail: 1160389236@qq.com
基金资助:
LIU Yajun1( ), DUAN Yipeng2, LI Rongfu1, CHI Zeyong1, WU Yongming1,2,*(
), DUAN Yipeng2, LI Rongfu1, CHI Zeyong1, WU Yongming1,2,*( )
)
Received:2024-11-25
Online:2025-06-18
Published:2025-06-11
摘要:
入湖河口区域水文和地形环境复杂,碳、氮等营养物质伴随固体颗粒物在不同区域差异化沉积,为底栖生物提供了多样生境。以饶河入湖河口表层沉积物为研究对象,通过高通量测序技术探究不同养分环境下原核生物群落结构及其胞外酶活性特征。结果表明,沉积物养分含量主要影响原核生物群落的Beta多样性而不是Alpha多样性,高营养环境下(SOC>15 g·kg−1)原核生物群落组成更加接近,其Beta多样性显著高于中营养(SOC 10-15 g·kg−1)和低营养环境(SOC<10 g·kg−1)(p<0.05)。对于优势(Top10)古菌和细菌属,发现Methanosaeta、Candidatus_Methanomethylicus、MBNT15、SC-I-84和Anaeromyxobacter受主要养分(SOC、TN、TP和NH4+-N)的正向影响,在高营养环境下大量富集;而Candidatus_Nitrosotenuis和Latescibacterota则相反(p<0.05)。同时发现,β-葡萄糖苷酶、酸性磷酸酶、脲酶、亮氨酸氨基肽酶和亚硝酸还原酶与SOC、TN、TP和NH4+-N均呈显著正相关关系,在高营养环境下表现出更高的酶活性;而过氧化物酶则相反,在低营养环境下活性更强(p<0.05)。该研究深入分析了沉积物养分变化对原核生物群落结构与功能的影响,强调了具有不同养分含量的入湖河口沉积物对于维持水生态系统原核生物生境和功能多样性至关重要。
中图分类号:
刘亚军, 段亦鹏, 李荣富, 池泽涌, 吴永明. 不同养分环境下入湖河口沉积物原核生物群落特征——以饶河入湖口为例[J]. 生态环境学报, 2025, 34(6): 853-862.
LIU Yajun, DUAN Yipeng, LI Rongfu, CHI Zeyong, WU Yongming. Characteristics of Prokaryotic Communities in Sediments of a Lake Inflow Estuary Under Different Nutrient Environments: A Case Study of the Raohe Estuary[J]. Ecology and Environmental Sciences, 2025, 34(6): 853-862.
| 取样点 | w(Clay)/% | w(Silt)/% | w(Sand)/% | pH | w(SOC)/(g·kg‒1) | w(TN)/(g·kg‒1) | w(TP)/(g·kg‒1) | w(NO3‒-N)/(mg·kg‒1) | w(NH4+-N)/(mg·kg‒1) | 样点分组 |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 17.49 | 25.80 | 56.71 | 6.52 | 10.78 | 0.63 | 0.44 | 0.58 | 15.47 | M |
| S2 | 16.49 | 24.21 | 59.30 | 6.44 | 14.98 | 0.83 | 0.49 | 0.85 | 28.09 | M |
| S3 | 12.49 | 20.21 | 67.30 | 6.59 | 8.90 | 0.43 | 0.35 | 0.15 | 22.32 | L |
| S4 | 10.48 | 8.12 | 81.40 | 6.63 | 4.71 | 0.18 | 0.25 | 0.36 | 0.47 | L |
| S5 | 26.50 | 36.26 | 37.24 | 6.44 | 14.59 | 0.99 | 0.46 | 0.44 | 26.97 | M |
| S6 | 6.47 | 4.01 | 89.52 | 6.93 | 1.87 | 0.10 | 0.19 | 0.23 | 0.20 | L |
| S7 | 4.47 | 2.05 | 93.48 | 7.08 | 1.18 | 0.10 | 0.20 | 0.39 | 0.15 | L |
| S8 | 20.51 | 44.27 | 35.22 | 6.51 | 18.20 | 1.20 | 0.58 | 0.70 | 36.97 | H |
| S9 | 21.50 | 33.28 | 45.22 | 6.46 | 19.27 | 1.38 | 0.63 | 1.50 | 42.22 | H |
| S10 | 20.51 | 38.21 | 41.28 | 6.31 | 19.00 | 1.37 | 0.56 | 1.03 | 45.91 | H |
| S11 | 13.49 | 23.13 | 63.38 | 6.75 | 10.01 | 0.72 | 0.48 | 0.99 | 13.88 | M |
| S12 | 14.48 | 14.14 | 71.38 | 6.70 | 7.69 | 0.64 | 0.37 | 0.63 | 13.14 | L |
| S13 | 20.50 | 32.26 | 47.24 | 6.67 | 20.22 | 1.32 | 0.58 | 0.47 | 32.88 | H |
| S14 | 17.49 | 19.21 | 63.30 | 6.61 | 15.50 | 0.99 | 0.53 | 0.56 | 40.52 | H |
| S15 | 17.49 | 19.21 | 63.30 | 6.54 | 13.30 | 0.84 | 0.47 | 0.15 | 12.90 | M |
表1 样点分组及理化参数
Table 1 Sample group and physicochemical parameters
| 取样点 | w(Clay)/% | w(Silt)/% | w(Sand)/% | pH | w(SOC)/(g·kg‒1) | w(TN)/(g·kg‒1) | w(TP)/(g·kg‒1) | w(NO3‒-N)/(mg·kg‒1) | w(NH4+-N)/(mg·kg‒1) | 样点分组 |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 17.49 | 25.80 | 56.71 | 6.52 | 10.78 | 0.63 | 0.44 | 0.58 | 15.47 | M |
| S2 | 16.49 | 24.21 | 59.30 | 6.44 | 14.98 | 0.83 | 0.49 | 0.85 | 28.09 | M |
| S3 | 12.49 | 20.21 | 67.30 | 6.59 | 8.90 | 0.43 | 0.35 | 0.15 | 22.32 | L |
| S4 | 10.48 | 8.12 | 81.40 | 6.63 | 4.71 | 0.18 | 0.25 | 0.36 | 0.47 | L |
| S5 | 26.50 | 36.26 | 37.24 | 6.44 | 14.59 | 0.99 | 0.46 | 0.44 | 26.97 | M |
| S6 | 6.47 | 4.01 | 89.52 | 6.93 | 1.87 | 0.10 | 0.19 | 0.23 | 0.20 | L |
| S7 | 4.47 | 2.05 | 93.48 | 7.08 | 1.18 | 0.10 | 0.20 | 0.39 | 0.15 | L |
| S8 | 20.51 | 44.27 | 35.22 | 6.51 | 18.20 | 1.20 | 0.58 | 0.70 | 36.97 | H |
| S9 | 21.50 | 33.28 | 45.22 | 6.46 | 19.27 | 1.38 | 0.63 | 1.50 | 42.22 | H |
| S10 | 20.51 | 38.21 | 41.28 | 6.31 | 19.00 | 1.37 | 0.56 | 1.03 | 45.91 | H |
| S11 | 13.49 | 23.13 | 63.38 | 6.75 | 10.01 | 0.72 | 0.48 | 0.99 | 13.88 | M |
| S12 | 14.48 | 14.14 | 71.38 | 6.70 | 7.69 | 0.64 | 0.37 | 0.63 | 13.14 | L |
| S13 | 20.50 | 32.26 | 47.24 | 6.67 | 20.22 | 1.32 | 0.58 | 0.47 | 32.88 | H |
| S14 | 17.49 | 19.21 | 63.30 | 6.61 | 15.50 | 0.99 | 0.53 | 0.56 | 40.52 | H |
| S15 | 17.49 | 19.21 | 63.30 | 6.54 | 13.30 | 0.84 | 0.47 | 0.15 | 12.90 | M |
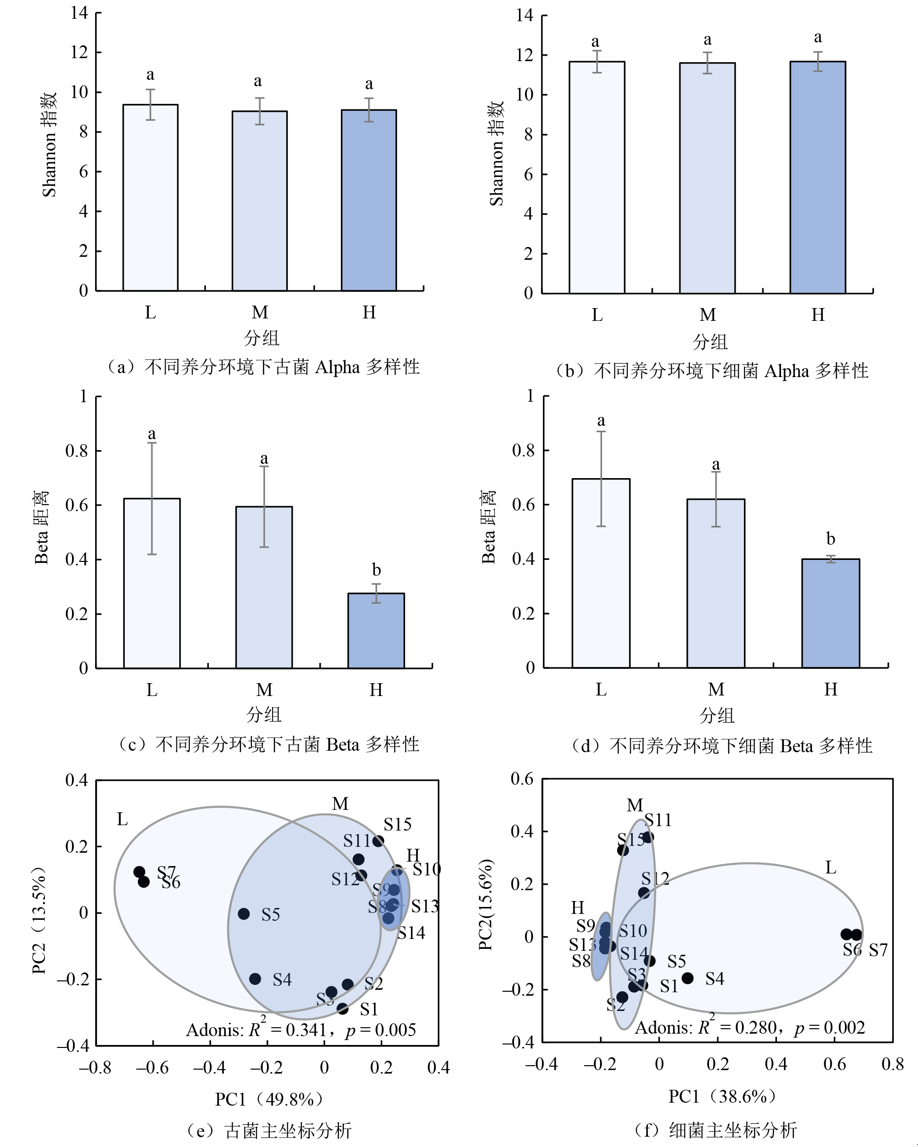
图2 不同养分环境下沉积物原核生物多样性 不同小写字母表示不同分组在p<0.05水平差异显著;Adonis多元方差分析不同分组因素对样品差异的解释度(其中r2越大分组间差异越大;p<0.05时表示差异显著)
Figure 2 Sediment prokaryotic biodiversity in different nutrient environments
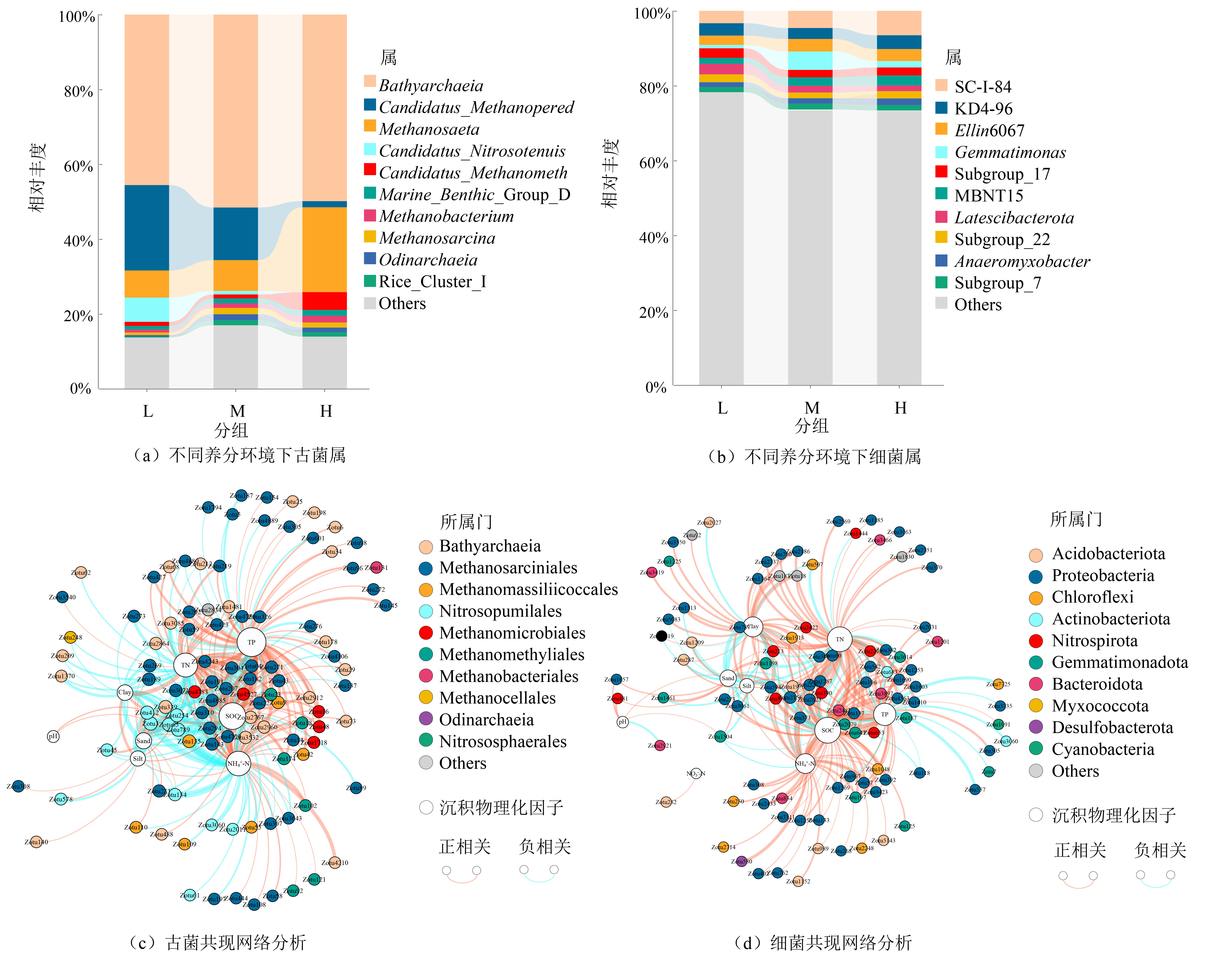
图3 不同养分环境下沉积物原生生物群落组成特征 显示强相关性(Spearman’s |r|>0.8)和显著性(p<0.01),节点大小与连接数成正比,条线粗细与相关度成正比
Figure 3 Characteristics of community composition of sediment protists in different nutrient environments
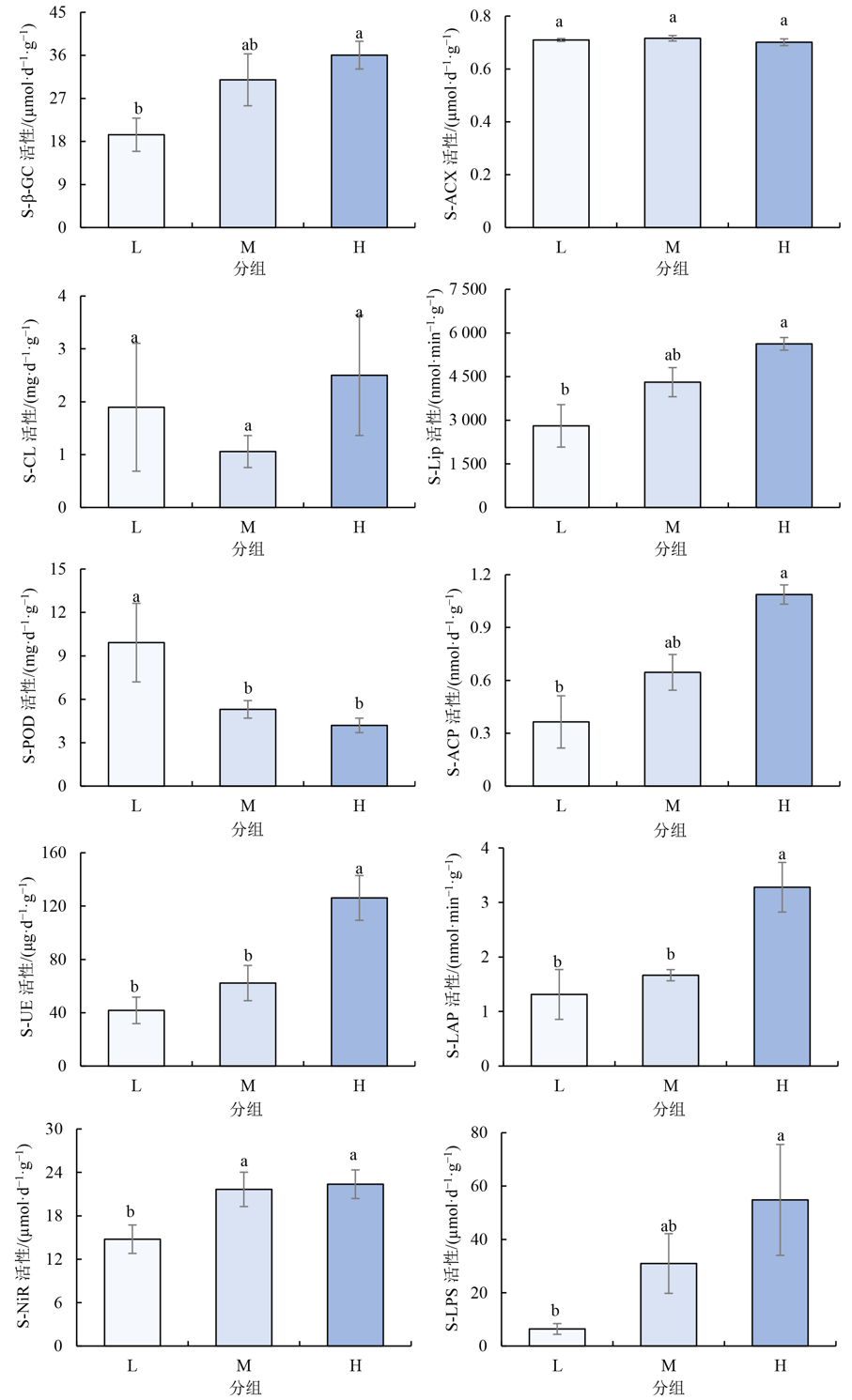
图5 不同养分环境下沉积物胞外酶活性特征 S-β-GC:β-葡萄糖苷酶;S-ACX:酸性木聚糖苷酶;S-CL:纤维素酶;S-Lip:木质素过氧化物酶;S-POD:过氧化物酶;S-ACP:酸性磷酸酶;S-UE:脲酶;S-LAP:亮氨酸氨基肽酶;S-NiR:亚硝酸还原酶;S-LPS:脂肪酶。不同小写字母表示不同分组在p<0.05水平差异显著
Figure 5 Characteristics of extracellular enzyme activity in sediments under different nutrient environments
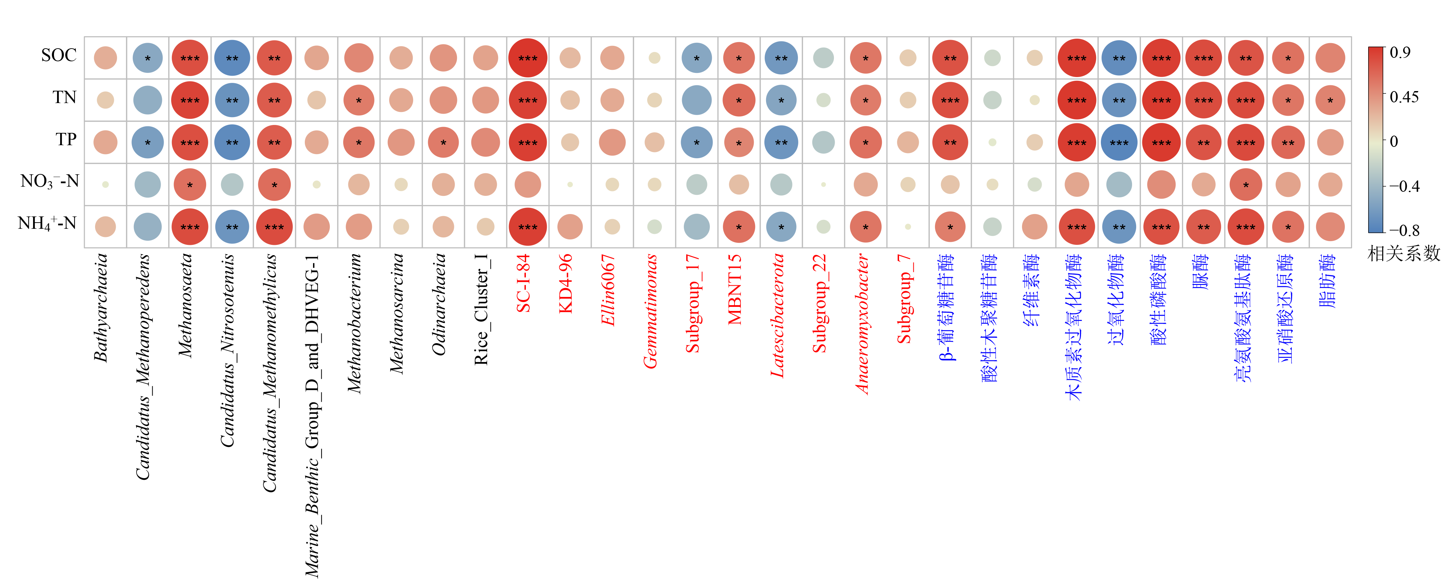
图6 沉积物养分与原核生物及胞外酶活性相关性分析 SOC:有机碳;TN:总氮;TP:总磷;NO3--N:硝氮;NH4+-N:氨氮;* p<0.05水平上显著相关;** p<0.01水平上显著相关;***p<0.001水平上显著相关
Figure 6 Correlation analysis of sediment nutrients, prokaryotic communities, and extracellular enzyme activities
| [1] |
AN J X, LIU C, WANG Q, et al., 2019. Soil bacterial community structure in Chinese wetlands[J]. Geoderma, 337: 290-299.
DOI |
| [2] | BASTIDA F, ELDRIDGE D J, GARCÍA C, et al., 2021. Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes[J]. The ISME Journal, 15(7): 2081-2091. |
| [3] | BEGMATOV S, BELETSKY A V, DEDYSH S N, et al., 2022. Genome analysis of the candidate phylum MBNT15 bacterium from a boreal peatland predicted its respiratory versatility and dissimilatory iron metabolism[J]. Frontiers in Microbiology, 13: 951761. |
| [4] | BODMER P, WILKINSON J, LORKE A, 2020. Sediment properties drive spatial variability of potential methane production and oxidation in small streams[J]. Journal of Geophysical Research: Biogeosciences, 125(1): e2019JG005213. |
| [5] | CHANG W J, SUN J L, PANG Y, et al., 2020. Effects of different habitats on the bacterial community composition in the water and sediments of Lake Taihu, China[J]. Environmental Science and Pollution Research, 27(36): 44983-44994. |
| [6] | CHI Y, ZHAO M W, SUN J K, et al., 2019. Mapping soil total nitrogen in an estuarine area with high landscape fragmentation using a multiple-scale approach[J]. Geoderma, 339(5): 70-84. |
| [7] | CRUMP B C, BOWEN J L, 2024. The microbial ecology of estuarine ecosystems[J]. Annual Review of Marine Science, 16: 335-360. |
| [8] | DAI Z J, MEI X F, DARBY S E, et al., 2018. Fluvial sediment transfer in the Changjiang (Yangtze) river-estuary depositional system[J]. Journal of Hydrology, 566: 719-734. |
| [9] | GENG Y Q, WANG D W, YANG W B, 2017. Effects of different inundation periods on soil enzyme activity in riparian zones in Lijiang[J]. Catena, 149(Part 1): 19-27. |
| [10] |
GUPTA V V S R, TIEDJE J M, 2024. Ranking environmental and edaphic attributes driving soil microbial community structure and activity with special attention to spatial and temporal scales[J]. mLife, 3(1): 21-41.
DOI PMID |
| [11] |
HAN X G, SCHUBERT C J, FISKAL A, et al., 2020. Eutrophication as a driver of microbial community structure in lake sediments[J]. Environmental Microbiology, 22(8): 3446-3462.
DOI PMID |
| [12] | JI N N, LIU Y, WANG S R, et al., 2022. Buffering effect of suspended particulate matter on phosphorus cycling during transport from rivers to lakes[J]. Water Research, 216: 118350. |
| [13] | JIAO S, PENG Z H, QI J J, et al., 2021. Linking bacterial-fungal relationships to microbial diversity and soil nutrient cycling[J]. mSystems, 6(2): e01052-20. |
| [14] | JUNG M-Y, SEDLACEK C J, KITS K D, et al., 2022. Ammonia-oxidizing archaea possess a wide range of cellular ammonia affinities[J]. The ISME Journal, 16(1): 272-283. |
| [15] | KRAUSE L, RODIONOV A, SCHWEIZER S A, et al., 2018. Microaggregate stability and storage of organic carbon is affected by clay content in arable Luvisols[J]. Soil and Tillage Research, 182: 123-129. |
| [16] | LI Q C, WANG L L, FU Y, et al., 2023. Transformation of soil organic matter subjected to environmental disturbance and preservation of organic matter bound to soil minerals: A review[J]. Journal of Soils and Sediments, 23(3): 1485-1500. |
| [17] | LI S L, GANG D, ZHAO S J, et al., 2020. Response of ammonia oxidation activities to water-level fluctuations in riparian zones in a column experiment[J]. Chemosphere, 269: 128702. |
| [18] | LIAO W F, TONG D, LI Z W, et al., 2021. Characteristics of microbial community composition and its relationship with carbon, nitrogen and sulfur in sediments[J]. Science of The Total Environment, 795: 148848. |
| [19] | LIU R X, YAO Y, CHU Q W, et al., 2024. Enhanced soil microbial stability is associated with soil organic carbon storage under high-altitude forestation[J]. Journal of Environmental Management, 370: 122462. |
| [20] | LIU Y J, WEN Z, HE M J, et al., 2023. Dry-wet seasonal variations of microbially mediated carbon metabolism in soils of a floodplain lake[J]. Ecohydrology, 16(3): e2510. |
| [21] | MARTIN M, 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads[J]. EMBnet. Journal, 17(1): 10-12. |
| [22] | NIEKERK L V, ADAMS J, JAMES N C, et al., 2020. An estuary ecosystem classification that encompasses biogeography and a high diversity of types in support of protection and management[J]. African Journal of Aquatic Science, 45(1-2): 199-216. |
| [23] | OLIVEIRA R S, PINTO O H B, QUIRINO B F, et al., 2023. Genome-resolved metagenomic analysis of Great Amazon Reef System sponge-associated Latescibacterota bacteria and their potential contributions to the host sponge and reef[J]. Frontiers in Microbiomes, 2: 1206961. |
| [24] | ONLEY JENNY R, AHSAN S, SANFORD ROBERT A, et al., 2018. Denitrification by anaeromyxobacter dehalogenans, a common soil bacterium lacking the nitrite reductase genes nirS and nirK[J]. Applied and Environmental Microbiology, 84(4): e01985-17. |
| [25] | PARK K, KIM C Y, KIRK M F, et al., 2023. Effects of natural non-volcanic CO2 leakage on soil microbial community composition and diversity[J]. Science of The Total Environment, 862: 160754. |
| [26] | PRAETZEL L S E, PLENTER N, SCHILLING S, et al., 2020. Organic matter and sediment properties determine in-lake variability of sediment CO2 and CH4 production and emissions of a small and shallow lake[J]. Biogeosciences, 17(20): 5057-5078. |
| [27] | RAKHSH F, GOLCHIN A, BEHESHTI AL AGHA A, et al., 2020. Mineralization of organic carbon and formation of microbial biomass in soil: Effects of clay content and composition and the mechanisms involved[J]. Soil Biology and Biochemistry, 151: 108036. |
| [28] | REED H E, MARTINY J B H, 2013. Microbial composition affects the functioning of estuarine sediments[J]. The ISME Journal, 7(4): 868-879. |
| [29] | SANTMIRE J A, LEFF L G, 2007. The influence of stream sediment particle size on bacterial abundance and community composition[J]. Aquatic Ecology, 41(2): 153-160. |
| [30] | TIAN L, SHI W, 2014. Soil peroxidase regulates organic matter decomposition through improving the accessibility of reducing sugars and amino acids[J]. Biology and Fertility of Soils, 50(5): 785-794. |
| [31] | UNDA-CALVO J, MARTÍNEZ-SANTOS M, RUIZ-ROMERA E, et al., 2019. Implications of denitrification in the ecological status of an urban river using enzymatic activities in sediments as an indicator[J]. Journal of Environmental Sciences, 75: 255-268. |
| [32] | VANWONTERGHEM I, EVANS P N, PARKS D H, et al., 2016. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota[J]. Nature Microbiology, 1(12): 16170. |
| [33] |
WAGG C, BENDER S F, WIDMER F, et al., 2014. Soil biodiversity and soil community composition determine ecosystem multifunctionality[J]. Proceedings of the National Academy of Sciences of the United States of America, 111(14): 5266-5270.
DOI PMID |
| [34] | WANG J W, CHEN Y, CAI P G, et al., 2022. Impacts of municipal wastewater treatment plant discharge on microbial community structure and function of the receiving river in Northwest Tibetan Plateau[J]. Journal of Hazardous Materials, 423(Part B): 127170. |
| [35] | XU Q C, ZHANG H, VANDENKOORNHUYSE P, et al., 2024. Carbon starvation raises capacities in bacterial antibiotic resistance and viral auxiliary carbon metabolism in soils[J]. Proceedings of the National Academy of Sciences, 121(16): e2318160121. |
| [36] | XU S, CAI C, GUO J H, et al., 2018. Different clusters of Candidatus ‘Methanoperedens nitroreducens’-like archaea as revealed by high-throughput sequencing with new primers[J]. Scientific Reports, 8(1): 7695. |
| [37] | YANG J Q, ZHANG X N, BOURG I C, et al., 2021b. 4D imaging reveals mechanisms of clay-carbon protection and release[J]. Nature Communications, 12(1): 622. |
| [38] | YANG J, JIANG H C, SUN X X, et al., 2021a. Distinct co-occurrence patterns of prokaryotic community between the waters and sediments in lakes with different salinity[J]. FEMS Microbiology Ecology, 97(1): fiaa234. |
| [39] | YUAN C B, ZHAO F C, ZHAO X H, et al., 2020. Woodchips as sustained-release carbon source to enhance the nitrogen transformation of low C/N wastewater in a baffle subsurface flow constructed wetland[J]. Chemical Engineering Journal, 392: 124840. |
| [40] | YUAN Y Q, LI X Z, XIE Z L, et al., 2022. Annual lateral organic carbon exchange between salt marsh and adjacent water: A case study of east headland marshes at the Yangtze Estuary[J]. Frontiers in Marine Science, 8: 809618. |
| [41] | ZHANG F, ZHANG H, YUAN Y, et al., 2020. Different response of bacterial community to the changes of nutrients and pollutants in sediments from an urban river network[J]. Frontiers of Environmental Science & Engineering, 14(2): 28. |
| [42] | ZHANG H, JIANG N, ZHANG S Y, et al., 2024. Soil bacterial community composition is altered more by soil nutrient availability than pH following long-term nutrient addition in a temperate steppe[J]. Frontiers in Microbiology, 15: 1455891. |
| [43] | ZHANG S, HU W J, XU Y, et al., 2022. Linking bacterial and fungal assemblages to soil nutrient cycling within different aggregate sizes in agroecosystem[J]. Frontiers in Microbiology, 13: 1038536. |
| [44] | ZHOU J, YOU X G, NIU B W, et al., 2020. Enhancement of methanogenic activity in anaerobic digestion of high solids sludge by nano zero-valent iron[J]. Science of The Total Environment, 703: 135532. |
| [1] | 欧阳美凤, 尹宇莹, 张金谌, 刘清霖, 谢意南, 方平. 洞庭湖典型水域重金属含量的空间分布与来源解析[J]. 生态环境学报, 2024, 33(8): 1269-1278. |
| [2] | 卢聪. 生物炭负载纳米零价铁对沉积物中十溴二苯乙烷去除效果及机制[J]. 生态环境学报, 2024, 33(8): 1279-1288. |
| [3] | 李多美, 孔涛, 陈曦, 高明夫, 高熙梣, 曾泽宇, 保佳慧. 古龙酸母液混制肥和草席覆盖措施对新疆旱区牧草生长和土壤养分含量的影响[J]. 生态环境学报, 2024, 33(4): 548-559. |
| [4] | 姜晓静, 谢嘉慧, 马凯, 高丽. 天鹅湖沉积物中解磷菌的解磷能力及其对硬毛藻生长的影响[J]. 生态环境学报, 2024, 33(4): 633-644. |
| [5] | 梁川, 杨艳芳, 俞姗姗, 周利, 张经纬, 张秀娟. 围网与围塘养鱼下沉积物微生物量和群落结构特征差异[J]. 生态环境学报, 2023, 32(8): 1487-1495. |
| [6] | 童永杰, 汪毅, 华玉妹, 赵建伟, 刘广龙, 蒋永参. 有机电子供体影响下硝酸盐和铁对磷转化的驱动作用[J]. 生态环境学报, 2023, 32(7): 1263-1274. |
| [7] | 张广毅, 张嘉涛, 王晓伟. 湖泊底泥微生物燃料电池中磷形态分布及释放研究[J]. 生态环境学报, 2023, 32(3): 590-598. |
| [8] | 杨奇丽, 窦韦丽, 刘之文, 郭景, 吕刚. 正构烷烃示源的阜新细河河道石油烃类污染特征及其影响因素分析[J]. 生态环境学报, 2023, 32(3): 599-608. |
| [9] | 何文宣, 李垒, 孙思宇, 李昌, 李久义, 田秀君. 北运河水体、沉积物和鱼类中微塑料的分布特征研究[J]. 生态环境学报, 2023, 32(11): 1901-1912. |
| [10] | 梁川, 杨艳芳, 俞姗姗, 周利, 张经纬, 张秀娟. 围网与围塘养鱼下沉积物微生物量和群落结构特征差异[J]. 生态环境学报, 2023, 32(10): 1802-1810. |
| [11] | 吉冰静, 刘艺, 吴杨, 高淑涛, 曾祥英, 于志强. 长江口及邻近东海沉积物中多环芳烃和含氧多环芳烃的分布特征、来源及生态风险[J]. 生态环境学报, 2022, 31(7): 1400-1408. |
| [12] | 包宇飞, 胡明明, 王殿常, 吴兴华, 王雨春, 李姗泽, 王启文, 温洁. 黄柏河梯级水库沉积物营养盐与重金属分布特征及污染评价[J]. 生态环境学报, 2021, 30(5): 1005-1016. |
| [13] | 张凯, 郭紫微, 王倩, 韩雅, 李贶家, 张中帅. 华中地区水库型水源地抗生素抗性细菌的赋存特征研究[J]. 生态环境学报, 2021, 30(5): 1017-1022. |
| [14] | 解旭东, 侯智昊, 李楠, 岳翠霞, 李雅, 杨方社. 中国胡焕庸线下方四区域沉积物和土壤中抗生素污染特征及生态风险评价[J]. 生态环境学报, 2021, 30(5): 1023-1033. |
| [15] | 余楚, 李剑锋, 吕敦玉. 大兴安岭南段某矿区河流表层沉积物重金属污染及风险评价[J]. 生态环境学报, 2021, 30(11): 2223-2231. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
