生态环境学报 ›› 2024, Vol. 33 ›› Issue (8): 1279-1288.DOI: 10.16258/j.cnki.1674-5906.2024.08.012
收稿日期:2024-05-09
出版日期:2024-08-18
发布日期:2024-09-25
作者简介:卢聪(1988年生),男,高级工程师,博士,主要从事污染控制技术研究。E-mail: lucong_1988@163.com
基金资助:Received:2024-05-09
Online:2024-08-18
Published:2024-09-25
摘要:
新型溴代阻燃剂十溴二苯乙烷(DBDPE)已经成为目前最常用的溴代阻燃剂。随着DBDPE在各种环境介质中被普遍检测到,其环境污染和治理正引起广泛的关注。迄今为止,生物炭负载零价铁材料(nZVI/BC)去除沉积物中DBDPE的应用尚未见报道。利用甘蔗渣通过液相还原法制备nZVI/BC,研究nZVI/BC去除沉积物体系里DBDPE的动力学过程,并探究其作用机制,为利用碳基零价铁去除沉积物中DBDPE提供了科学依据和技术支持。研究发现增加材料投加量、含水量、反应温度和降低底物初始质量分数,均能提高DBDPE的去除效率。高剂量的nZVI/BC能提供更多的吸附位点,并增大与DBDPE的接触面积,从而吸附更多的DBDPE分子。DBDPE质量分数的增加,将进一步增加DBDPE分子间的竞争反应,导致DBDPE分子与nZVI/BC颗粒的接触率降低。大量的水可以提供更多的活性H,从而提高与DBDPE反应的机会。温度升高,分子运动增强,提高了nZVI/BC与DBDPE的接触频率,也提高了nZVI的反应活性。SEM表征结果显示,生物炭(BC)的加入使纳米零价铁(nZVI)均匀分散在生物炭的表面,改善了nZVI的分散程度,提高了nZVI的反应活性。红外吸收光谱FTIR检测结果表明反应后nZVI/BC表面的−OH的伸缩振动和−CH2−的弯曲强度均减弱,Fe−CO特征峰消失,并且出现了C=C的伸缩振动峰。nZVI/BC的微孔及介孔结构和表面的Fe−O、−OH、−CH2−、C−O、C−H、C=O和−COOH等基团均能为DBDPE提供吸附位点,且DBDPE苯环结构上的π电子可能与nZVI/BC表面上的阳离子形成π−电荷相互作用。GC-ECNI-MS检测结果表明DBDPE被nZVI/BC降解过程中可能脱溴生成八溴二苯乙烷,ECOSAR (Ecological Structure Activity Relationships)软件预测nZVI/BC对DBDPE的降解降低了母体的生物毒性。
中图分类号:
卢聪. 生物炭负载纳米零价铁对沉积物中十溴二苯乙烷去除效果及机制[J]. 生态环境学报, 2024, 33(8): 1279-1288.
LU Cong. Removal Effect and Mechanism of DBDPE in Sediments by Biochar-loaded Nano-zero-valent Iron[J]. Ecology and Environment, 2024, 33(8): 1279-1288.
| 投加量/ (g∙g−1) | 初始质量分数/ (mg∙kg−1) | 温度/ ℃ | 沉积物与水 质量比 | k1/ h−1 | r2 |
|---|---|---|---|---|---|
| 0.02 | 10 | 25 | 1∶2 | 0.0064 | 0.659 |
| 0.04 | 10 | 25 | 1∶2 | 0.0089 | 0.633 |
| 0.08 | 10 | 25 | 1∶2 | 0.0117 | 0.652 |
| 0.10 | 10 | 20 | 1∶2 | 0.0159 | 0.830 |
| 0.10 | 10 | 25 | 1∶2 | 0.0190 | 0.793 |
| 0.10 | 10 | 30 | 1∶2 | 0.0260 | 0.821 |
| 0.10 | 15 | 25 | 1∶2 | 0.0138 | 0.724 |
| 0.10 | 20 | 25 | 1∶2 | 0.0118 | 0.787 |
| 0.10 | 10 | 25 | 2∶1 | ‒ | ‒ |
| 0.10 | 10 | 25 | 1∶1 | 0.0108 | 0.749 |
| 0.10 | 10 | 25 | 1∶3 | 0.0224 | 0.848 |
表1 不同影响因素条件下nZVI/BC对沉积物中DBDPE去除的动力学参数
Table 1 Kinetics parameters of DBDPE removal by nZVI/BC under different influencing factors in sediment
| 投加量/ (g∙g−1) | 初始质量分数/ (mg∙kg−1) | 温度/ ℃ | 沉积物与水 质量比 | k1/ h−1 | r2 |
|---|---|---|---|---|---|
| 0.02 | 10 | 25 | 1∶2 | 0.0064 | 0.659 |
| 0.04 | 10 | 25 | 1∶2 | 0.0089 | 0.633 |
| 0.08 | 10 | 25 | 1∶2 | 0.0117 | 0.652 |
| 0.10 | 10 | 20 | 1∶2 | 0.0159 | 0.830 |
| 0.10 | 10 | 25 | 1∶2 | 0.0190 | 0.793 |
| 0.10 | 10 | 30 | 1∶2 | 0.0260 | 0.821 |
| 0.10 | 15 | 25 | 1∶2 | 0.0138 | 0.724 |
| 0.10 | 20 | 25 | 1∶2 | 0.0118 | 0.787 |
| 0.10 | 10 | 25 | 2∶1 | ‒ | ‒ |
| 0.10 | 10 | 25 | 1∶1 | 0.0108 | 0.749 |
| 0.10 | 10 | 25 | 1∶3 | 0.0224 | 0.848 |
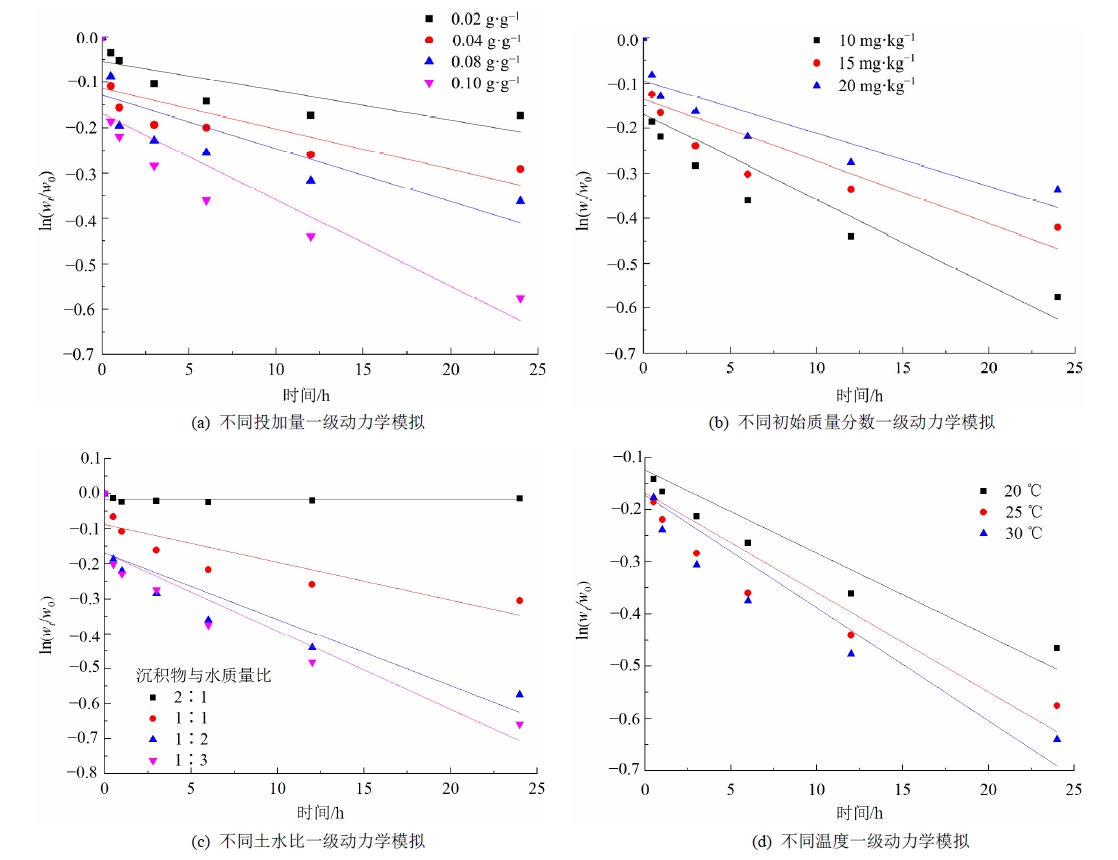
图6 不同影响因素下nZVI/BC对沉积物中DBDPE去除的准一级动力学模拟
Figure 6 The pseudo-first-order kinetics model of DBDPE removal by nZVI/BCunder different influencing factors in sediment
| 化合物 | 急性毒性/(mg∙L−1) | 慢性毒性/(mg∙L−1) | |||||
|---|---|---|---|---|---|---|---|
| 鱼 (LC50) | 绿藻 (EC50) | 水蚤 (LC50) | 鱼 (ChV) | 绿藻 (ChV) | 水蚤 (ChV) | ||
| DBDPE | 2.8×10−8 | 3.0×10−6 | 4.7×10−8 | 9.8×10−9 | 8.5×10−6 | 9.1×10−8 | |
| Octa-BDPE | 6.9×10−6 | 2.0×10−4 | 8.9×10−6 | 1.8×10−6 | 3.2×10−4 | 8.5×10−6 | |
表2 ECOSAR模型预测DBDPE及其降解产物的急性和慢性毒性
Table 2 Acute and chronic toxicity of DBDPE and its degradation products using ECOSAR software
| 化合物 | 急性毒性/(mg∙L−1) | 慢性毒性/(mg∙L−1) | |||||
|---|---|---|---|---|---|---|---|
| 鱼 (LC50) | 绿藻 (EC50) | 水蚤 (LC50) | 鱼 (ChV) | 绿藻 (ChV) | 水蚤 (ChV) | ||
| DBDPE | 2.8×10−8 | 3.0×10−6 | 4.7×10−8 | 9.8×10−9 | 8.5×10−6 | 9.1×10−8 | |
| Octa-BDPE | 6.9×10−6 | 2.0×10−4 | 8.9×10−6 | 1.8×10−6 | 3.2×10−4 | 8.5×10−6 | |
| [1] |
AHMAD M, RAJAPAKSHA A U, LIM J E, et al., 2014. Biochar as a sorbent for contaminant management in soil and water: A review[J]. Chemosphere, 99: 19-33.
DOI PMID |
| [2] | BIANCO F, RACE M, PAPIRIO S, et al., 2021. The addition of biochar as a sustainable strategy for the remediation of PAH-contaminated sediments[J]. Chemosphere, 263: 128274. |
| [3] |
COVACI A, HARRAD S, ABDALLAH M A, et al., 2011. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour[J]. Environment International, 37(2): 532-556.
DOI PMID |
| [4] | DENG J M, DONG H R, ZHANG C, et al., 2018. Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine[J]. Separation and Purification Technology, 202: 130-137. |
| [5] |
FAN Z X, ZHANG Q, GAO B, et al., 2019. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention[J]. Chemosphere, 217: 85-94.
DOI PMID |
| [6] | HOU R, LIN L, LI H X, et al., 2021. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments: A critical review[J]. Water Research, 198: 117168. |
| [7] |
INYANG M, DICKENSON E, 2015. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review[J]. Chemosphere, 134: 232-240.
DOI PMID |
| [8] | ISHAG A, LI Y, ZHANG N, et al., 2020. Environmental application of emerging zero-valent iron-based materials on removal of radionuclides from the wastewater: A review[J]. Environmental Research, 188: 109855. |
| [9] | JI L J, WAN Y Q, ZHENG S R, et al., 2011. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption[J]. Environmental Science & Technology, 45(13): 5580-5586. |
| [10] | LING A Y, LU C, PENG C, et al., 2021. Characteristics of legacy and novel brominated flame retardants in water and sediment surrounding two e-waste dismantling regions in Taizhou, eastern China[J]. Science of the Total Environment, 794: 148744. |
| [11] |
LING X F, LI J S, ZHU W, et al., 2012. Synthesis of nanoscale zero-valent iron/ordered mesoporous carbon for adsorption and synergistic reduction of nitrobenzene[J]. Chemosphere, 87(6): 655-660.
DOI PMID |
| [12] | LIU J, LIU A R, WANG W, et al., 2019. Feasibility of nanoscale zero-valent iron (nZVI) for enhanced biological treatment of organic dyes[J]. Chemosphere, 237: 124470. |
| [13] | LIU Z G, ZHANG F S, WU J Z, 2010. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment[J]. Fuel, 89(2): 510-514. |
| [14] | LU C, WAN J, CHEN X, et al., 2022. Removal of decabromodiphenyl ethane (DBDPE) by BC/nZVI in the soil: Kinetics, pathways and mechanisms[J]. Journal of Environmental Chemical Engineering, 10(1): 107004. |
| [15] | MALETIC S, ISAKOVSKI M K, SIGMUND G, et al., 2022. Comparing biochar and hydrochar for reducing the risk of organic contaminants in polluted river sediments used for growing energy crops[J]. Science of the Total Environment, 843: 157122. |
| [16] | MEI G X, KUMAR H, HUANG H, et al., 2021. Desiccation cracks mitigation using biomass derived carbon produced from aquatic species in South China Sea[J]. Waste Biomass Valorization, 12(3): 1493-1505. |
| [17] | NASCIMENTO C T, VIEIRA M G A, SCHEUFELE F B, et al., 2022. Adsorption of atrazine from aqueous systems on chemically activated biochar produced from corn straw[J]. Journal of Environmental Chemical Engineering, 10(1): 107039. |
| [18] | PENG P, LANG Y H, WANG X M, 2016. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms[J]. Ecological Engineering, 90: 225-233. |
| [19] | RANGABHASHIYAM S, LINS P V D, OLIVERIRA L, et al., 2022. Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: A critical review[J]. Environmental Pollution, 293: 118581. |
| [20] |
SHEN K H, LI L, LIU J Z, et al., 2019. Stocks, flows and emissions of DBDPE in China and its international distribution through products and waste[J]. Environmental Pollution, 250: 79-86.
DOI PMID |
| [21] |
SHI T, CHEN S J, LUO X J, et al., 2009. Occurrence of brominated flame retardants other than polybrominated diphenyl ethers in environmental and biota samples from southern China[J]. Chemosphere, 74(7): 910-916.
DOI PMID |
| [22] | SHIH Y H, TAI Y T, 2010. Reaction of decabrominated diphenyl ether by zerovalent iron nanoplarticles[J]. Chemosphere, 78(10): 1200-1206. |
| [23] | SU L H, CHEN M, ZHUO G H, et al., 2021. Comparison of biochar materials derived from coconut husks and various types of livestock manure, and their potential for use in removal of H2S from biogas[J]. Sustainability, 13(11): 6262. |
| [24] |
SUN Y Q, YU I K M, TSANG D C W, et al., 2019. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater[J]. Environment International, 124: 521-532.
DOI PMID |
| [25] | WANG Y W, LING S Y, LU C, et al., 2020. Exploring the environmental fate of novel brominated flame retardants in a sediment-water-mudsnail system: Enrichment, removal, metabolism and structural damage[J]. Environmental Pollution, 265(Part B): 114924. |
| [26] | WIJITKOSUM S, 2022. Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications[J]. International Soil Water Conservation Research, 10(2): 335-341. |
| [27] |
WU F C, GUO J Y, CHANG H, et al., 2012. Polybrominated diphenyl ethers and decabromodiphenylethane in sediments from twelve lakes in China[J]. Environmental Pollution, 162: 262-268.
DOI PMID |
| [28] |
YAN J C, QIAN L B, GAO W G, et al., 2017. Enhanced Fenton-like degradation of trichloroethylene by hydrogen peroxide activated with nanoscale zero valent iron loaded on biochar[J]. Scientific Reports, 7: 43051.
DOI PMID |
| [29] |
ZHANG P, SUN H W, YU L, et al., 2013. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars[J]. Journal of Hazardous Materials, 244-245: 217-224.
DOI PMID |
| [30] | ZHU F, LI L W, REN W T, et al., 2017. Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr(VI) in the soil leachate by nZVI/Ni bimetal material[J]. Environmental Pollution, 227: 444-450. |
| [31] | 陈潇, 2020. 生物炭负载零价纳米铁去除土壤中十溴二苯乙烷及机制研究[D]. 上海: 华东理工大学. |
| CHEN X, 2020. Insight into removal of decabromodiphenyl ethane by biochar supported zero-valent-nano iron and mechanism in soil system[D]. Shanghai: East China University of Science and Technology. | |
| [32] | 李春娟, 2009. 芬顿法和类芬顿法对水中污染物的去除研究[D]. 哈尔滨: 哈尔滨工业大学. |
| LI C J, 2009. Investigation of removal of contaminants in water by fenton and fenton-like oxidation[D]. Harbin:Harbin Institute of Technology. |
| [1] | 陈文哲, 黄秋香, 孟凡德, 高金妍, 李敏, 张恩俊, 袁国栋. 草酸和酒石酸对稻田土壤中砷解吸行为的影响研究[J]. 生态环境学报, 2024, 33(8): 1298-1305. |
| [2] | 欧阳美凤, 尹宇莹, 张金谌, 刘清霖, 谢意南, 方平. 洞庭湖典型水域重金属含量的空间分布与来源解析[J]. 生态环境学报, 2024, 33(8): 1269-1278. |
| [3] | 赵乐依, 朱雪强, 刘健, 路平. 碳球负载纳米零价铁活化过硫酸盐降解水中恩诺沙星的性能研究[J]. 生态环境学报, 2024, 33(5): 757-770. |
| [4] | 王室苹, 李梅, 安娅, 秦好丽. 镁改性增强小麦秸秆生物炭对镉的吸附能力:表面络合模型研究[J]. 生态环境学报, 2024, 33(4): 617-625. |
| [5] | 姜晓静, 谢嘉慧, 马凯, 高丽. 天鹅湖沉积物中解磷菌的解磷能力及其对硬毛藻生长的影响[J]. 生态环境学报, 2024, 33(4): 633-644. |
| [6] | 肖江, 李晓刚, 赵博, 陈岩, 陈光才. 微纳富磷生物炭对土壤-苏柳系统中Cu和Pb稳定性的影响[J]. 生态环境学报, 2024, 33(3): 439-449. |
| [7] | 李高帆, 徐文卓, 卫昊明, 晏再生, 尤佳, 江和龙, 黄娟. 三维多孔生物炭吸附剂的制备及其对菲的吸附行为[J]. 生态环境学报, 2024, 33(2): 261-271. |
| [8] | 丛鑫, 曹平, 王晓博. 生物炭负载纳米铁活化过硫酸盐去除土壤中的五氯联苯[J]. 生态环境学报, 2024, 33(2): 282-290. |
| [9] | 梁川, 杨艳芳, 俞姗姗, 周利, 张经纬, 张秀娟. 围网与围塘养鱼下沉积物微生物量和群落结构特征差异[J]. 生态环境学报, 2023, 32(8): 1487-1495. |
| [10] | 童永杰, 汪毅, 华玉妹, 赵建伟, 刘广龙, 蒋永参. 有机电子供体影响下硝酸盐和铁对磷转化的驱动作用[J]. 生态环境学报, 2023, 32(7): 1263-1274. |
| [11] | 赵维彬, 唐丽, 王松, 刘玲玲, 王树凤, 肖江, 陈光才. 两种生物炭对滨海盐碱土的改良效果[J]. 生态环境学报, 2023, 32(4): 678-686. |
| [12] | 张广毅, 张嘉涛, 王晓伟. 湖泊底泥微生物燃料电池中磷形态分布及释放研究[J]. 生态环境学报, 2023, 32(3): 590-598. |
| [13] | 杨奇丽, 窦韦丽, 刘之文, 郭景, 吕刚. 正构烷烃示源的阜新细河河道石油烃类污染特征及其影响因素分析[J]. 生态环境学报, 2023, 32(3): 599-608. |
| [14] | 李卓轩, 彭自然, 何文辉, 卫瑞璐, 高琳茜. 羊粪炭对水体氮磷吸附条件的响应面优化及吸附机理研究[J]. 生态环境学报, 2023, 32(12): 2216-2227. |
| [15] | 苏丹, 罗桥冰, 董昱杉, 杨彩霞, 王鑫. 混合型生物炭对寒冷地区PAHs污染土壤微生物修复的强化作用[J]. 生态环境学报, 2023, 32(11): 1942-1951. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

