生态环境学报 ›› 2022, Vol. 31 ›› Issue (8): 1537-1546.DOI: 10.16258/j.cnki.1674-5906.2022.08.005
收稿日期:2021-10-20
出版日期:2022-08-18
发布日期:2022-10-10
通讯作者:
* 柴宝峰(1967年生),男,教授,博士,主要从事微生物生态学研究。E-mail: bfchai@sxu.edu.cn作者简介:王礼霄(1988年生),女,博士研究生,主要从事微生物生态学研究。E-mail: 525079118@qq.com
基金资助:
WANG Lixiao( ), LIU Jinxian, CHAI Baofeng*(
), LIU Jinxian, CHAI Baofeng*( )
)
Received:2021-10-20
Online:2022-08-18
Published:2022-10-10
摘要:
退耕还草是生态恢复的重要措施,地上植被演替是驱动微生物变化的最重要因素之一。在生态系统相对脆弱的华北亚高山草甸,微生物群落结构和氮循环的潜在功能受退耕还草的影响尚不清楚。利用16S rRNA基因的Illumina MiSeq测序和qPCR技术,研究了山西省宁武云中山亚高山退耕还草区不同恢复年限(农田、15 a、20 a、30 a)的草地土壤细菌群落结构及其氮循环功能基因的变化特征。结果表明,农田和不同恢复年限的草甸地上植被多样性指数(Shannon index)和丰富度指数(Richness index)以及土壤理化性质(土壤总碳、总氮、碳氮比、硝态氮、铵态氮和土壤含水率)和酶活性(过氧化氢酶、脲酶和蔗糖酶)差异显著(P<0.05)。优势细菌类群是放线菌门(Actinobacteria)、变形菌门(Proteobacteria)和酸杆菌门(Acidobacteria),土壤细菌群落多样性沿退耕时间序列显著增加,结构也有显著差异。土壤总碳、总氮、碳氮比与铵态氮含量是细菌群落结构变化的重要驱动因子(P<0.05)。植物群落与细菌群落的α多样性显著相关(P<0.05)。参与氮循环的功能基因nifH、amoA-AOA、amoA-AOB的丰度随自然恢复时间而增加(P<0.05)。可见,亚高山草地自然恢复过程中植被多样性、土壤理化性质、土壤细菌群落结构与氮循环相关功能发生了显著的变化。研究结果将为华北亚高山退耕还草区生态保护措施的制定提供数据支持。
中图分类号:
王礼霄, 刘晋仙, 柴宝峰. 华北亚高山土壤细菌群落及氮循环对退耕还草的响应[J]. 生态环境学报, 2022, 31(8): 1537-1546.
WANG Lixiao, LIU Jinxian, CHAI Baofeng. Response of Soil Bacterial Community and Nitrogen Cycle during the Natural Recovery of Abandoned Farmland in Subalpine of the North China[J]. Ecology and Environment, 2022, 31(8): 1537-1546.
| 参数 Parameters | Farmland | R15-year | R20-year | R30-year |
|---|---|---|---|---|
| 土壤酸碱度Soil pH | 6.54±0.16a | 6.47±0.03a | 6.41±0.04a | 6.58±0.10a |
| 土壤含水量Soil water content/% | 16.87±2.85b | 24.98±0.85a | 27.43±1.14a | 29.24±0.47a |
| 总氮Total nitrogen/% | 0.33±0.03a | 0.15±0.01c | 0.17±0.01c | 0.25±0.01b |
| 总碳Total carbon/% | 3.74±0.35a | 1.66±0.09c | 1.82±0.09c | 2.91±0.06b |
| 碳氮比carbon-nitrogen ratio | 11.42±0.11ab | 10.92±0.23b | 10.89±0.23b | 11.79±0.18a |
| 铵态氮NH4+-N/(g∙kg-1) | 1.32±0.05a | 0.45±0.0.03b | 0.42±0.01b | 0.42±0.01b |
| 硝态氮NO3--N/(g∙kg-1) | 0.38±0.01a | 0.19±0.04b | 0.16±0.03b | 0.15±0.02b |
| 过氧化氢酶 Catalase/mg∙(g∙20 min)-1 | 1.32±0.04a | 1.38±0.06a | 1.28±0.10a | 0.48±0.03b |
| 脲酶 Urease/[mg∙(g∙24 h)-1] | 1.62±0.01a | 0.61±0.02b | 0.61±0.01b | 0.49±0.03c |
| 蔗糖酶 Sucrase/[mg∙(g∙24 h)-1] | 0.33±0.01b | 0.51±0.06bc | 0.47±0.03c | 0.83±0.06a |
| 植物香农指数 Plant Shannon index | — | 2.45±0.03a | 2.14±0.09b | 2.05±0.07b |
| 植物丰富度指数 Plant richness index | — | 15.33±0.88a | 12.00±0.58b | 10.33±0.67b |
| 植物均匀度指数 Plant evenness index | — | 0.76±0.02a | 0.71±0.03a | 0.76±0.03a |
| 优势种 Dominant species | — | 嵩草 (Kobresia bellardii)、苔草 (Carex spp.)、委陵菜 (Potentilla chinensis Ser.) | 委陵菜 (Potentilla chinensis Ser.)、车前 (Plantago asiatica L.)、嵩草 (Kobresia bellardii) | 米口袋 (Gueldenstaedtia verna( Georgi) Boriss)、嵩草 (Kobresia bellardii)、车前 (Plantago asiatica L.) |
表1 农田与不同恢复年限草地土壤理化性质与植物群落参数
Table 1 Soil physicochemical properties and plant community parameters under different revegetation habitats and farmland
| 参数 Parameters | Farmland | R15-year | R20-year | R30-year |
|---|---|---|---|---|
| 土壤酸碱度Soil pH | 6.54±0.16a | 6.47±0.03a | 6.41±0.04a | 6.58±0.10a |
| 土壤含水量Soil water content/% | 16.87±2.85b | 24.98±0.85a | 27.43±1.14a | 29.24±0.47a |
| 总氮Total nitrogen/% | 0.33±0.03a | 0.15±0.01c | 0.17±0.01c | 0.25±0.01b |
| 总碳Total carbon/% | 3.74±0.35a | 1.66±0.09c | 1.82±0.09c | 2.91±0.06b |
| 碳氮比carbon-nitrogen ratio | 11.42±0.11ab | 10.92±0.23b | 10.89±0.23b | 11.79±0.18a |
| 铵态氮NH4+-N/(g∙kg-1) | 1.32±0.05a | 0.45±0.0.03b | 0.42±0.01b | 0.42±0.01b |
| 硝态氮NO3--N/(g∙kg-1) | 0.38±0.01a | 0.19±0.04b | 0.16±0.03b | 0.15±0.02b |
| 过氧化氢酶 Catalase/mg∙(g∙20 min)-1 | 1.32±0.04a | 1.38±0.06a | 1.28±0.10a | 0.48±0.03b |
| 脲酶 Urease/[mg∙(g∙24 h)-1] | 1.62±0.01a | 0.61±0.02b | 0.61±0.01b | 0.49±0.03c |
| 蔗糖酶 Sucrase/[mg∙(g∙24 h)-1] | 0.33±0.01b | 0.51±0.06bc | 0.47±0.03c | 0.83±0.06a |
| 植物香农指数 Plant Shannon index | — | 2.45±0.03a | 2.14±0.09b | 2.05±0.07b |
| 植物丰富度指数 Plant richness index | — | 15.33±0.88a | 12.00±0.58b | 10.33±0.67b |
| 植物均匀度指数 Plant evenness index | — | 0.76±0.02a | 0.71±0.03a | 0.76±0.03a |
| 优势种 Dominant species | — | 嵩草 (Kobresia bellardii)、苔草 (Carex spp.)、委陵菜 (Potentilla chinensis Ser.) | 委陵菜 (Potentilla chinensis Ser.)、车前 (Plantago asiatica L.)、嵩草 (Kobresia bellardii) | 米口袋 (Gueldenstaedtia verna( Georgi) Boriss)、嵩草 (Kobresia bellardii)、车前 (Plantago asiatica L.) |
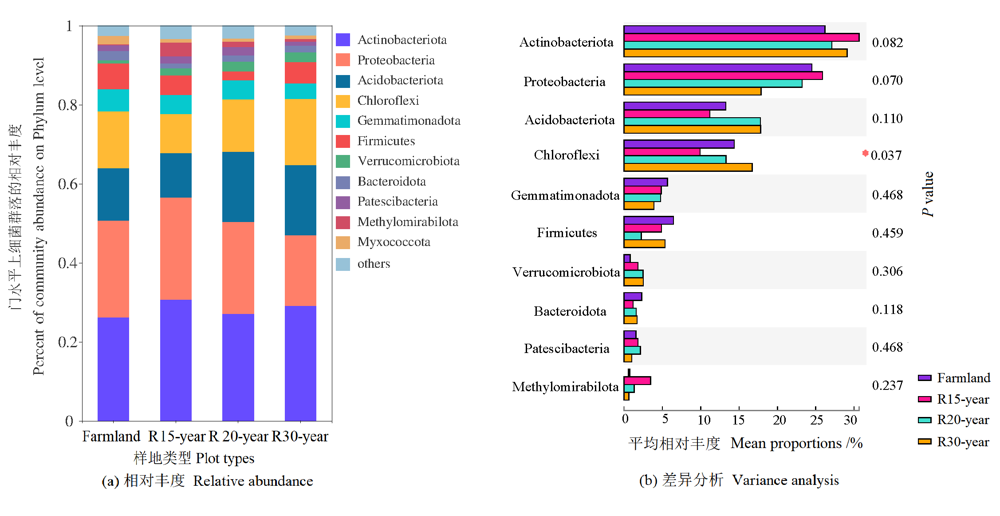
图1 农田和不同退耕年限草地细菌群落在门水平的相对丰度及差异分析 n=3。*代表P<0.05;**代表P<0.01。下同
Figure 1 The relative abundance of dominant bacteria phylum and variation analysis from different revegetation habitats and farmland n=3. * indicates significant differences at the 0.05 level; ** indicates significant differences at the 0.01 level. The same below
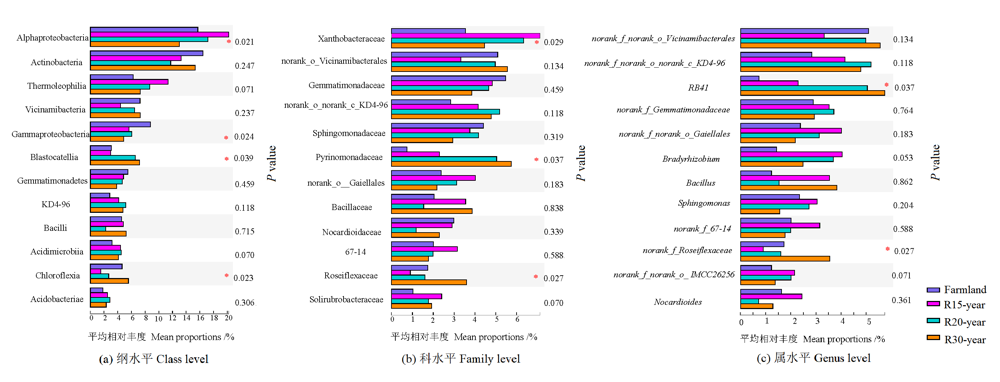
图2 农田和不同退耕年限草地细菌群落在纲、科、属水平的差异分析
Figure 2 The variation analysis of dominant bacteria class, family and genus from different revegetation habitats and farmland
| 样地类型 Plot types | 多样性指数Diversity index | ||||
|---|---|---|---|---|---|
| sobs指数 sobs | 香农指数Shannon index | 辛普森指数Simpson index | ACE指数 ACE index | 超1指数 Chao1 index | |
| Farmland | 2382.00±104.24a | 6.60±0.07a | 0.003±0.000c | 3173.08±160.56a | 3200.63±130.02a |
| R15-year | 1807.33±98.79c | 5.84±0.19c | 0.010±0.003a | 2555.52±101.41c | 2496.17±97.03b |
| R20-year | 2019.33±63.75bc | 6.13±0.03bc | 0.007±0.000ab | 2764.29±140.75ab | 2774.28±138.28b |
| R30-year | 2143.00±65.04ab | 6.27±0.05ab | 0.006±0.000ab | 2882.00±104.76ab | 2858.61±74.51ab |
表2 农田和不同恢复年限草地土壤细菌群落多样性
Table 2 Diversity of bacteria communities of different revegetation habitats and farmland
| 样地类型 Plot types | 多样性指数Diversity index | ||||
|---|---|---|---|---|---|
| sobs指数 sobs | 香农指数Shannon index | 辛普森指数Simpson index | ACE指数 ACE index | 超1指数 Chao1 index | |
| Farmland | 2382.00±104.24a | 6.60±0.07a | 0.003±0.000c | 3173.08±160.56a | 3200.63±130.02a |
| R15-year | 1807.33±98.79c | 5.84±0.19c | 0.010±0.003a | 2555.52±101.41c | 2496.17±97.03b |
| R20-year | 2019.33±63.75bc | 6.13±0.03bc | 0.007±0.000ab | 2764.29±140.75ab | 2774.28±138.28b |
| R30-year | 2143.00±65.04ab | 6.27±0.05ab | 0.006±0.000ab | 2882.00±104.76ab | 2858.61±74.51ab |
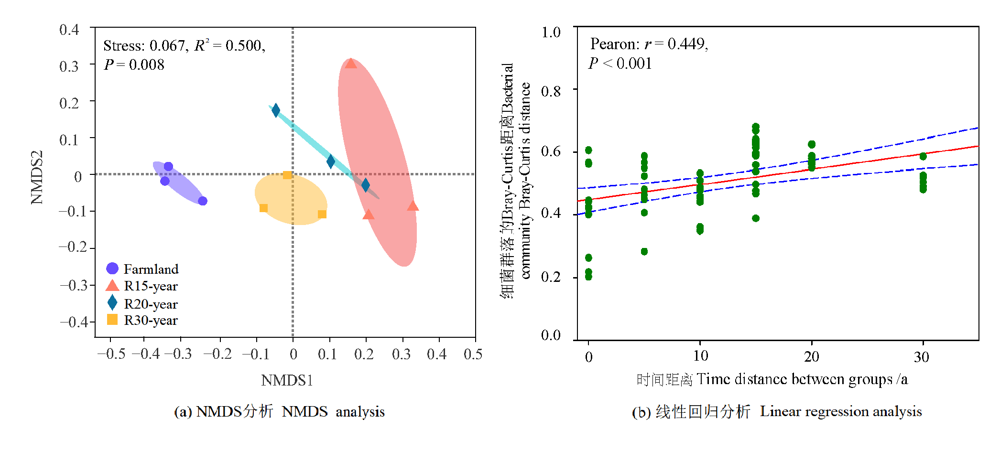
图3 农田和不同恢复年限草地细菌群落基于Bray-Curtis距离的NMDS分析及其与线性回归分析
Figure 3 NMDS and linear regression analysis of bacteria communities based on Bray-Curtis distance among different revegetation habitats and farmland
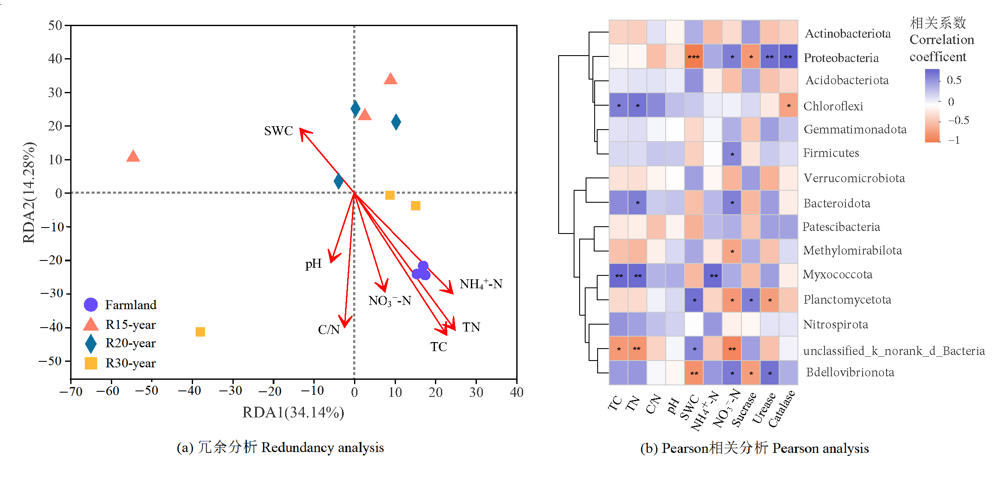
图4 农田和不同恢复年限草地细菌群落组成的冗余分析及优势门与环境因子的Pearson相关分析
Figure 4 Redundancy analysis of bacterial community composition and Pearson analysis with dominant phylum in different revegetation grasslands and farmland
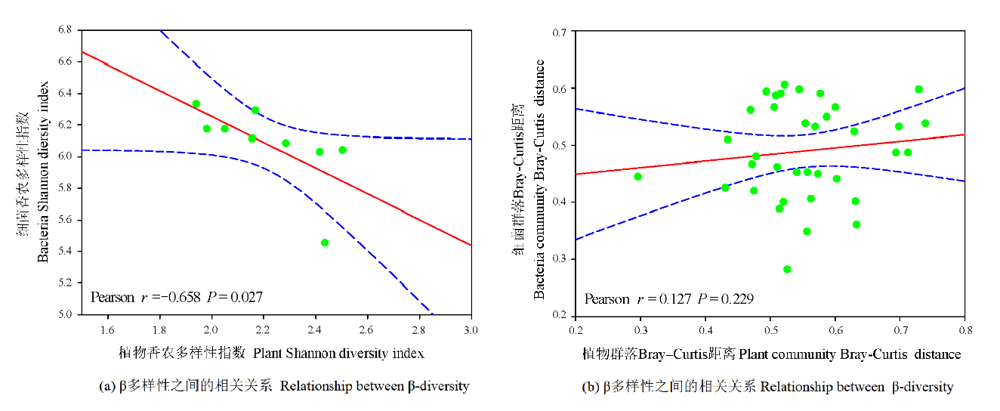
图5 农田和不同恢复年限草地土壤细菌群落与植物群落α多样性和β多样性之间的相关关系(基于Bray-Curtis距离)
Figure 5 Relationship between plant and soil bacterial α-diversity and β-diversity (based on Bray-Curtis distances) in different revegetation grasslands and farmland
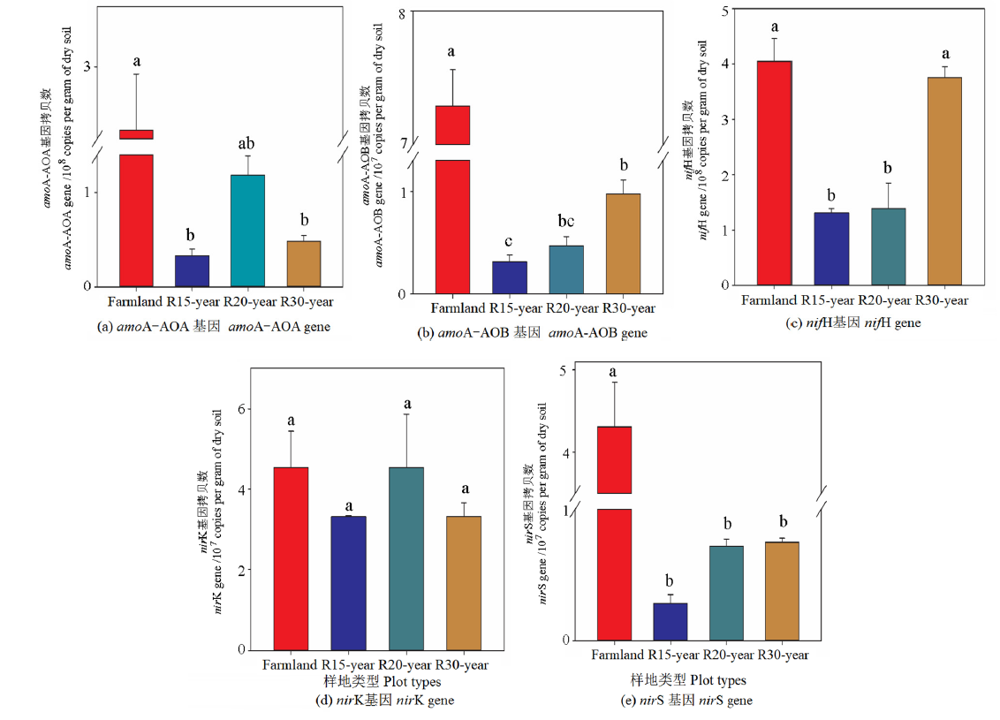
图6 农田和不同恢复年限草地参与氮循环主要过程的功能基因拷贝数(amoA-AOA, amoA-AOB, nifH, nirK and nirS) n=3,不同的字母表示两组数据差异显著P<0.05
Figure 6 Gene copy numbers of functional genes involved in major steps of the nitrogen cycle (amoA-AOA, amoA-AOB, nifH, nirK and nirS) in different revegetation grasslands and farmland n=3; different lowercase letters indicate significant differences P<0.05
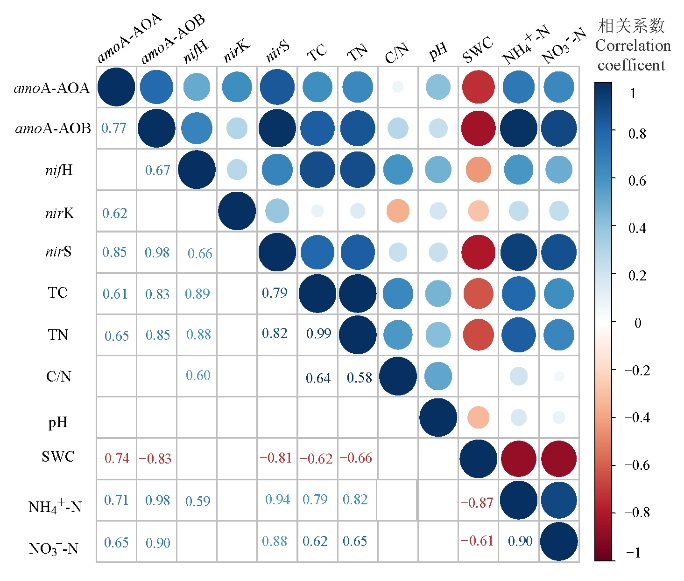
图7 参与氮循环主要过程的功能基因与土壤理化性质的相关关系
Figure 7 Correlations between gene copy numbers of functional genes involving in nitrogen cycling and soil physicochemical properties
| 参数 Parameter | Actinobacteriota | Proteobacteria | Acidobacteriota | Chloroflexi | Gemmatimonadota | Firmicutes | Verrucomicrobiota | Bacteroidota | Patescibacteria | Methylomirabilota | Myxococcota |
|---|---|---|---|---|---|---|---|---|---|---|---|
| amoA-AOA | 0.063 | 0.324 | 0.544 | 0.736** | 0.365 | 0.227 | -0.185 | 0.834** | 0.111 | -0.330 | 0.684* |
| AmoB-AOB | 0.275 | 0.420 | 0.285 | 0.742** | 0.524 | 0.499 | -0.395 | 0.839** | 0.093 | -0.371 | 0.953** |
| nifH | -0.252 | -0.163 | 0.211 | 0.551 | -0.090 | 0.050 | -0.137 | 0.539 | -0.193 | -0.513 | 0.528 |
| nirK | -0.169 | 0.143 | 0.671 | 0.528 | -0.037 | -0.116 | 0.288 | 0.62229* | 0.448 | -0.302 | 0.251 |
| nirS | 0.252 | 0.404 | 0.405 | 0.811** | 0.510 | 0.455 | -0.320 | 0.854** | 0.073 | -0.373 | 0.903** |
表3 参与氮循环主要过程的功能基因与优势门的相关关系
Table 3 Correlation between dominant phylum and functional genes involved in the main processes of nitrogen cycling
| 参数 Parameter | Actinobacteriota | Proteobacteria | Acidobacteriota | Chloroflexi | Gemmatimonadota | Firmicutes | Verrucomicrobiota | Bacteroidota | Patescibacteria | Methylomirabilota | Myxococcota |
|---|---|---|---|---|---|---|---|---|---|---|---|
| amoA-AOA | 0.063 | 0.324 | 0.544 | 0.736** | 0.365 | 0.227 | -0.185 | 0.834** | 0.111 | -0.330 | 0.684* |
| AmoB-AOB | 0.275 | 0.420 | 0.285 | 0.742** | 0.524 | 0.499 | -0.395 | 0.839** | 0.093 | -0.371 | 0.953** |
| nifH | -0.252 | -0.163 | 0.211 | 0.551 | -0.090 | 0.050 | -0.137 | 0.539 | -0.193 | -0.513 | 0.528 |
| nirK | -0.169 | 0.143 | 0.671 | 0.528 | -0.037 | -0.116 | 0.288 | 0.62229* | 0.448 | -0.302 | 0.251 |
| nirS | 0.252 | 0.404 | 0.405 | 0.811** | 0.510 | 0.455 | -0.320 | 0.854** | 0.073 | -0.373 | 0.903** |
| [1] |
BAI Z, YE J, WEI Y L, et al., 2021. Soil depth-dependent C/N stoichiometry and fungal and bacterial communities along a temperate forest succession gradient[J]. CATENA, DOI: 10.1016/j.catena.2021.105613.
DOI |
| [2] |
BARDGETT R D, PUTTEN W, 2014. Belowground biodiversity and ecosystem functioning[J]. Nature, 515(7528): 505-511.
DOI URL |
| [3] |
BIER R L, VOSS K A, BERNHARDT E S, 2014. Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams[J]. The ISME Journal, 9(6): 1378.
DOI URL |
| [4] |
BLAUD A, BAS V, MENON M, et al., 2017. The abundance of nitrogen cycle genes and potential greenhouse gas fluxes depends on land use type and little on soil aggregate size[J]. Applied Soil Ecology, 125: 1-11.
DOI URL |
| [5] |
CAMPBELL B J, POLSON S W, HANSON T E, et al., 2010. The effect of nutrient deposition on bacterial communities in Arctic tundra soil[J]. Environmental Microbiology, 12(7): 1842-1854.
DOI PMID |
| [6] |
CAO C Y, ZHANG Y, CUI Z B, et al., 2021. Recovery of Soil-Denitrifying Community along a Chronosequence of Sand-Fixation Forest in a Semi-Arid Desertified Grassland[J]. Forests, 12(3): 354.
DOI URL |
| [7] | DENG L, ZHANG Z N, SHANGGUAN Z, et al., 2014. Long-term fencing effects on plant diversity and soil properties in China[J]. Soil Tillage Researh, 137: 7-15. |
| [8] |
FAN M C, LIN Y B, HUO H B, et al., 2016. Microbial communities in riparian soils of a settling pond for mine drainage treatment[J]. Water Research, 96: 198-207.
DOI PMID |
| [9] |
HOSSAIN Z, SUGIYAMA S I, 2011. Geographical structure of soil microbial communities in northern Japan: Effects of distance, land use type and soil properties[J]. European Journal of Soil Biology, 47(2): 88-94.
DOI URL |
| [10] |
KARDOL P, BEZEMER T M, PUTTEN W, 2010. Temporal variation in plant-soil feedback controls succession[J]. Ecology Letters, 9(9): 1080-1088.
DOI URL |
| [11] | KURAMAE E, GAMPER H, VAN VEEN J, et al., 2011. Soil and plant factors driving the community of soil-borne microorganisms across chronosequences of secondary succession of chalk grasslands with a neutral pH[J]. FEMS Microbiology, 77(2): 285-294. |
| [12] |
KUYPERS M M M, MARCHANT H K, KARTAL BORAN, et al., 2018. The microbial nitrogen-cycling network[J]. Nature Reviews Microbiology, 16(5): 263-276.
DOI PMID |
| [13] |
LEE-CRUZ L, EDWARDS D P, TRIPATHI B M, et al., 2013. Impact of logging and forest conversion to oil palm plantations on soil bacterial communities in Borneo[J]. Applied and Environmental Microbiology, 79(23): 7290-7297.
DOI URL |
| [14] |
LI H, YE D D, WANG X G, et al., 2014. Soil bacterial communities of different natural forest types in Northeast China[J]. Plant and Soil, 383 (1-2): 203-216.
DOI URL |
| [15] |
LI Y Q, XU M, SUN O J, et al., 2004. Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests[J]. Soil Biology and Biochemistry, 36(12): 2111-2114.
DOI URL |
| [16] |
LIN Y T, WHITMAN W B, COLEMAN D C, et al., 2012. Comparison of soil bacterial communities between coastal and inland forests in a subtropical area[J]. Applied Soil Ecology, 60: 49-55.
DOI URL |
| [17] |
LOZANO Y M, HORTAL S, ARMAS C, et al., 2014. Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment[J]. Soil Biology and Biochemistry, 78: 298-306.
DOI URL |
| [18] | LUO Z, LIU J, JIA T, et al., 2020. Soil Bacterial Community Response and Nitrogen Cycling Variations Associated with Subalpine Meadow Degradation on the Loess Plateau, China[J]. Applied Environmental Microbiology, 86(9): e00180-20. |
| [19] |
MARTENS W, BERUBE P M, URAKAWA H, et al., 2009. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria[J]. Nature, 461(7266): 976-979.
DOI URL |
| [20] |
MATETSKAYA A Y, KARASYOVA T A, POPOVA N N, et al., 2021 Conservation and Restoration Prospects of Semi-Natural Plant Communities when Creating Parks in the Southern Russia's Steppe[J]. IOP Conference Series: Earth and Environmental Science, 817(1): 12065-12069.
DOI URL |
| [21] |
NACKE H, FISCHER C, THURMER A, et al., 2014. Land use type significantly affects microbial gene transcription in soil[J]. Microbial Ecology, 67(4): 919-930.
DOI PMID |
| [22] | NIE Y X, HAN X G, CHEN J, et al., 2019. Interrelationships among soil nitrogen transformation rates, functional gene abundance and soil properties in a tropical forest with exogenous N inputs[J]. Biogeosciences Discussions, 101: 1-29. |
| [23] |
SCHIMEL J, BALSER T C, WALLENSTEIN M, 2007, Microbial stress-response physiology and ITS implications for ecosystem function[J]. Ecology, 88(6): 1386-1394.
DOI PMID |
| [24] |
SCHMIDT J E, KENT A D, BRISSON V L, et al., 2019. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling[J]. Microbiome, 7(1): 146.
DOI PMID |
| [25] |
TANG Y Q, YU G R, ZHANG X Y, et al., 2018. Changes in nitrogen- cycling microbial communities with depth in temperate and subtropical forest soils[J]. Applied Soil Ecology, 124: 218-228.
DOI URL |
| [26] |
TSCHERKO D, HAMMESFAHR U, ZELTNEr G, et al., 2005. Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain[J]. Basic and Applied Ecology, 6(4): 367-383.
DOI URL |
| [27] |
WANG B, GUO B L, XUE S, et al., 2011. Changes in soil physico-chemical and microbiological properties during natural succession on abandoned farmland in the Loess Plateau[J]. Environmental Earth Sciences, 62(5): 915-925.
DOI URL |
| [28] |
WANG G L, LIU G B, XU M X, 2009. Above and belowground dynamics of plant community succession following abandonment of farmland on the Loess Plateau, China[J]. Plant and Soil, 322(1): 343.
DOI URL |
| [29] | WANG H H, LI X, LI X, et al., 2017. Changes of microbial population and N-cycling function genes with depth in three Chinese paddy soils[J]. PLoS One, 12(12): e189506. |
| [30] |
WANG Y Y, QI L, HUANG R, et al., 2020. Characterization of Denitrifying Community for Application in Reducing Nitrogen: A Comparison of nirK and nirS Gene Diversity and Abundance[J]. Applied Biochemistry Biotechnology, 192(1): 22-41.
DOI URL |
| [31] |
YANG Y F, WU L W, LIN Q Y, et al., 2013. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland[J]. Global Change Biology, 19(2): 637-648.
DOI PMID |
| [32] |
YAO M J, RUI J P, LI J B, et al., 2014. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe[J]. Soil Biology and Biochemistry, 79: 81-90.
DOI URL |
| [33] |
YUAN Y L, SI G C, WANG J, et al., 2014. Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan Plateau[J]. FEMS Microbiology Ecology, 87(1): 121-132.
DOI PMID |
| [34] |
ZHANG C, LIU G B, XUE S, et al., 2016. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau[J]. Soil Biology and Biochemistry, 97: 40-49.
DOI URL |
| [35] |
ZHANG Y G, ZHANG X Q, LIU X D, et al., 2007. Microarray-based analysis of changes in diversity of microbial genes involved in organic carbon decomposition following land use/cover changes[J]. FEMS Microbiology Letters, 266(2): 144-151.
PMID |
| [36] |
ZHONG Y Q W, YANG W M, WANG R W, et al., 2018. Decreased occurrence of carbon cycle functions in microbial communities along with long-term secondary succession[J]. Soil Biology and Biochemistry, 123: 207-217.
DOI URL |
| [37] |
ZUO X A, WANG S K, LÜ P, et al., 2016. Plant functional diversity enhances associations of soil fungal diversity with vegetation and soil in the restoration of semiarid sandy grassland[J]. Ecology and Evolution, 6(1): 318-328.
DOI PMID |
| [38] | 常庆瑞, 安韶山, 刘京, 等, 1999. 黄土高原恢复植被防止土地退化效益研究[J]. 土壤侵蚀与水土保持学报, 5(4): 6-9. |
| CHANG Q R, AN S S, LIU J, et al., 1999. Study on benefits of recovering vegetation to prevent land deterioration on Loess Plateau[J]. Journal of Soil Erosion and Soil and Water Conservation, 5(4): 6-9. | |
| [39] | 樊博, 林丽, 曹广民, 等, 2020. 不同演替状态下高寒草甸土壤物理性质与植物根系的相互关系[J]. 生态学报, 40(7): 2300-2309. |
| FAN B, LIN L, CAO G M, et al., 2020. Relationship between plant roots and physical soil properties in alpine meadows at different degradation stages[J]. Acta Ecologica Sinica, 40(7): 2300-2309. | |
| [40] | 海旭莹, 董凌勃, 汪晓珍, 等, 2020. 黄土高原退耕还草地C、N、P生态化学计量特征对植物多样性的影响[J]. 生态学报, 40(23): 8570-8581. |
| HAI X Y, DONG L B, WANG X Z, et al., 2020. Effects of carbon, nitrogen, and phosphorus ecological stoichiometry characteristics on plant diversity since returning farmland to grassland on the Loess Plateau[J]. Acta Ecologica Sinica, 40(23): 8570-8581. | |
| [41] | 贺纪正, 张丽梅, 2013. 土壤氮素转化的关键微生物过程及机制[J]. 微生物学通报, 40(1): 98-108. |
| HE J Z, ZHANG L M, 2013. Key processes and microbial mechanisms of soil nitrogen transformation[J]. Microbiology China, 40(1): 98-108. | |
| [42] | 史利江, 高杉, 姚晓军, 等, 2021. 晋西北黄土丘陵区不同植被恢复下的土壤碳氮累积特征[J]. 生态环境学报, 30(9): 1787-1796. |
| SHI L J, GAO S, YAO X J, et al., 2021. Characteristics of soil carbon and nitrogen accumulation under different vegetation restoration in the loess hilly region of Northwest Shanxi Province[J]. Ecology and Environmental Sciences, 30(9): 1787-1796. | |
| [43] |
王光华, 刘俊杰, 于镇华, 等, 2016. 土壤酸杆菌门细菌生态学研究进展[J]. 生物技术通报, 32(2): 14-20.
DOI |
| WANG G H, LIU J J, YU Z H, et al., 2016. Research progress of Acidobacteria ecology in soils[J]. Biotechnology Bulletin, 32(2): 14-20. | |
| [44] | 王杨, 2014. 不同酸度土壤硝化和反硝化活性的差异[D]. 大连: 大连交通大学: 7. |
| WANG Y, 2014. Difference in the activity of nitrification and denitrification with different soil acidity[D]. Dalian: Dalian Jiaotong University: 7. | |
| [45] | 徐白璐, 钟文辉, 黄欠如, 等, 2017. 长期施肥酸性旱地土壤硝化活性及自养硝化微生物特征[J]. 环境科学, 38(8): 3473-3482. |
| XU B L, ZHONG W H, HUANG Q R, et al., 2017. Nitrification activity and autotrophic nitrifiers in long-term fertilized acidic upland soils[J]. Environmental Science, 38(8): 3473-3482. | |
| [46] | 张文彦, 樊江文, 钟华平, 等, 2010. 中国典型草原优势植物功能群氮磷化学计量学特征研究[J]. 草地学报, 18(4): 503-509. |
| ZHANG W Y, FAN J W, ZHONG H P, et al., 2010. The nitrogen: phosphorus stoichiometry of different plant functional groups for dominant species of typical steppes in China[J]. Acta Agrestia Sinica, 18(4): 503-509. | |
| [47] | 赵鹏宇, 2019. 山西亚高山华北落叶松林土壤微生物群落构建机制[D]. 太原: 山西大学: 17-19. |
| ZHAO P Y, 2019. Assembly mechanism of soil microbial community in Larix principis-rupprechtii Mayr forest of subalpine north China in Shanxi Province[D]. Taiyuan: Shanxi University: 17-19. |
| [1] | 杜丹丹, 高瑞忠, 房丽晶, 谢龙梅. 旱区盐湖盆地土壤重金属空间变异及对土壤理化因子的响应[J]. 生态环境学报, 2023, 32(6): 1123-1132. |
| [2] | 李阳, 侯志勇, 陈薇, 于晓英, 谢永宏, 黄鑫, 谭佩阳, 李继承, 黎尚林, 杨辉. 大围山高山湿地植物多样性与区系组成研究[J]. 生态环境学报, 2023, 32(4): 643-650. |
| [3] | 王哲, 田胜尼, 张永梅, 张和禹, 周忠泽. 巢湖派河口滩涂植物群落特征研究[J]. 生态环境学报, 2022, 31(9): 1823-1831. |
| [4] | 王磊, 温远光, 周晓果, 朱宏光, 孙冬婧. 尾巨桉与红锥混交对林下植被和土壤性质的影响[J]. 生态环境学报, 2022, 31(7): 1340-1349. |
| [5] | 张博文, 秦娟, 任忠明, 陈子齐, 姚舜佳, 刘烨, 宋炎玉. 坡向对北亚热带区马尾松纯林及不同针阔混交林型林下植物多样性的影响[J]. 生态环境学报, 2022, 31(6): 1091-1100. |
| [6] | 段文军, 李达, 李冲. 5种不同林龄尾巨桉人工林林下植物多样性及其影响因素分析[J]. 生态环境学报, 2022, 31(5): 857-864. |
| [7] | 杨冲, 王春燕, 王文颖, 毛旭峰, 周华坤, 陈哲, 索南吉, 靳磊, 马华清. 青藏高原黄河源区高寒草地土壤营养特征变化及质量评价[J]. 生态环境学报, 2022, 31(5): 896-908. |
| [8] | 胡靓达, 周海菊, 黄永珍, 姚贤宇, 叶绍明, 喻素芳. 不同杉木林分类型植物多样性及其土壤碳氮关系的研究[J]. 生态环境学报, 2022, 31(3): 451-459. |
| [9] | 夏开, 邓鹏飞, 马锐豪, 王斐, 温正宇, 徐小牛. 马尾松次生林转换为湿地松和杉木林对土壤细菌群落结构和多样性的影响[J]. 生态环境学报, 2022, 31(3): 460-469. |
| [10] | 宋秀丽, 黄瑞龙, 柯彩杰, 黄蔚, 章武, 陶波. 不同种植方式对连作土壤细菌群落结构和多样性的影响[J]. 生态环境学报, 2022, 31(3): 487-496. |
| [11] | 玄锦, 李祖婵, 邹诚, 秦子博, 吴雅华, 黄柳菁. 江心洲景观类型和格局对植物多样性的多尺度影响——以闽江流域福州段为例[J]. 生态环境学报, 2022, 31(12): 2320-2330. |
| [12] | 刘佩伶, 刘效东, 冯英杰, 苏宇乔, 甘先华, 张卫强. 新丰江水库库区水源涵养林土壤饱和导水率特征[J]. 生态环境学报, 2022, 31(10): 1993-2001. |
| [13] | 陈双双, 朱宁华, 周光益, 袁星明, 尚海, 王迤翾. 不同等级石漠化环境下人工乔木林的植被与土壤物理特征[J]. 生态环境学报, 2022, 31(1): 52-61. |
| [14] | 王瑞, 宋祥云, 柳新伟. 黄河三角洲不同植被类型土壤酶活性的季节变化[J]. 生态环境学报, 2022, 31(1): 62-69. |
| [15] | 赵丽, 郭春燕, 张文军, 王晓江, 刘平生. 扎兰屯地区典型天然林群落特征及其相关性分析[J]. 生态环境学报, 2021, 30(7): 1353-1359. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
