生态环境学报 ›› 2022, Vol. 31 ›› Issue (3): 487-496.DOI: 10.16258/j.cnki.1674-5906.2022.03.007
宋秀丽1( ), 黄瑞龙1, 柯彩杰1, 黄蔚1, 章武1,*(
), 黄瑞龙1, 柯彩杰1, 黄蔚1, 章武1,*( ), 陶波2,*(
), 陶波2,*( )
)
收稿日期:2021-10-11
出版日期:2022-03-18
发布日期:2022-05-25
通讯作者:
陶波,教授,主要研究方向为植物保护。E-mail: botaol@163.com作者简介:宋秀丽(1984年生),女,讲师,博士,主要研究方向土壤资源与土壤生态。E-mail: songxiuli5251@163.com
基金资助:
SONG Xiuli1( ), HUANG Ruilong1, KE Caijie1, HUANG Wei1, ZHANG Wu1,*(
), HUANG Ruilong1, KE Caijie1, HUANG Wei1, ZHANG Wu1,*( ), TAO Bo2,*(
), TAO Bo2,*( )
)
Received:2021-10-11
Online:2022-03-18
Published:2022-05-25
摘要:
大豆连作对土壤微生物群落产生负面影响,轮作有利于土壤微生物群落多样性的形成,但关于不同轮作制度对连作土壤微生物群落结构和多样性的影响尚不明确。以东北黑土大豆连作土壤为研究对象,利用高通量测序技术,研究休耕(CK)、休耕-大豆(FS)、玉米-大豆(CS)、小麦-大豆(WS)和大豆连作(SC)5种种植方式对土壤细菌群落多样性的影响。结果表明,主成分分析显示前4种种植方式土壤细菌群落结构显著不同于SC,CK和FS土壤细菌群落结构相似,CS和WS土壤细菌群落结构相似。5种种植方式在土壤细菌Chao1指数、ACE指数和Shannon指数上呈现显著差异(P<0.05),其中FS处理细菌Chao1指数、ACE指数和Shannon指数显著最高,SC处理最低。由OTUs韦恩图分析可知,CK处理特有的OTUs数量最多,FS次之,CS最少。与SC相比,有益菌属硝化螺旋菌(Nitrospira)、厌氧蝇菌(Anaerolinea)、固氮菌(Azotobacter)及甲烷八叠球菌(Methanosarcina)相对丰度在FS土壤中显著增高,分别增加了0.47%、0.29%、0.37%和0.12%,芽孢杆菌(Bacills)相对丰度在WS中显著增高。不同种植方式的土壤化学性质呈现显著差异,并且土壤中有机质(OM)和有效磷(P)、铜(Cu)、锰(Mn)等化学性质与土壤细菌群落组成显著相关。综上,不同种植方式影响土壤细菌群落组成和多样性,不同种植方式下土壤化学性质的差异影响土壤细菌群落结构组成。该研究结果可为破除连作障碍,引导种植方式提供理论参考。
中图分类号:
宋秀丽, 黄瑞龙, 柯彩杰, 黄蔚, 章武, 陶波. 不同种植方式对连作土壤细菌群落结构和多样性的影响[J]. 生态环境学报, 2022, 31(3): 487-496.
SONG Xiuli, HUANG Ruilong, KE Caijie, HUANG Wei, ZHANG Wu, TAO Bo. Effects of Different Cropping Systems on Bacterial Community Structure and Diversity in Continuous Cropping Soil[J]. Ecology and Environment, 2022, 31(3): 487-496.
| 土壤化学性质 Soil chemical properties | 处理 Treatment | ||||
|---|---|---|---|---|---|
| CK | FS | CS | WS | SC | |
| w(OM)/(g∙kg-1) | 74.22±2.71a | 60.53±8.41ab | 54.12±1.44bc | 46.04±1.91c | 74.02±3.62a |
| w(N)/(mg∙kg-1) | 318±11.86b | 364±2.08ab | 311±7.54b | 248±8.39c | 387±37.54a |
| w(P)/(mg∙kg-1) | 29.33±0.88c | 12.33±0.88e | 38.33±1.20b | 47.33±0.88a | 25.00±0.58d |
| w(K)/(mg∙kg-1) | 313±13.22a | 228±0.58b | 152±11.32c | 231±2.52b | 323±1.76a |
| pH | 6.29±0.04a | 6.13±0.05b | 6.25±0.01a | 6.19±0.04ab | 6.24±0.01ab |
| w(Cu)/(mg∙kg-1) | 0.84±0.04c | 0.65±0.02d | 1.33±0.04b | 1.84±0.04a | 1.29±0.01b |
| w(Zn)/(mg∙kg-1) | 0.19±0.01c | 0.46±0.09b | 0.14±0.04c | 0.50±0.03b | 0.69±0.02a |
| w(Fe)/(mg∙kg-1) | 136.86±0.55d | 244±4.02b | 208±9.10c | 196±3.47c | 304±0.69a |
| w(Mn)/(mg∙kg-1) | 35.09±0.21a | 10.91±0.23e | 13.95±0.72d | 16.48±0.54c | 32.54±0.19b |
| w(B)/(mg∙kg-1) | 0.37±0.03ab | 0.30±0.00a | 1.02±0.64a | 0.96±0.11a | 0.70±0.27a |
表1 不同种植方式对土壤化学性质的影响
Table 1 Soil chemical properties of different cropping systems
| 土壤化学性质 Soil chemical properties | 处理 Treatment | ||||
|---|---|---|---|---|---|
| CK | FS | CS | WS | SC | |
| w(OM)/(g∙kg-1) | 74.22±2.71a | 60.53±8.41ab | 54.12±1.44bc | 46.04±1.91c | 74.02±3.62a |
| w(N)/(mg∙kg-1) | 318±11.86b | 364±2.08ab | 311±7.54b | 248±8.39c | 387±37.54a |
| w(P)/(mg∙kg-1) | 29.33±0.88c | 12.33±0.88e | 38.33±1.20b | 47.33±0.88a | 25.00±0.58d |
| w(K)/(mg∙kg-1) | 313±13.22a | 228±0.58b | 152±11.32c | 231±2.52b | 323±1.76a |
| pH | 6.29±0.04a | 6.13±0.05b | 6.25±0.01a | 6.19±0.04ab | 6.24±0.01ab |
| w(Cu)/(mg∙kg-1) | 0.84±0.04c | 0.65±0.02d | 1.33±0.04b | 1.84±0.04a | 1.29±0.01b |
| w(Zn)/(mg∙kg-1) | 0.19±0.01c | 0.46±0.09b | 0.14±0.04c | 0.50±0.03b | 0.69±0.02a |
| w(Fe)/(mg∙kg-1) | 136.86±0.55d | 244±4.02b | 208±9.10c | 196±3.47c | 304±0.69a |
| w(Mn)/(mg∙kg-1) | 35.09±0.21a | 10.91±0.23e | 13.95±0.72d | 16.48±0.54c | 32.54±0.19b |
| w(B)/(mg∙kg-1) | 0.37±0.03ab | 0.30±0.00a | 1.02±0.64a | 0.96±0.11a | 0.70±0.27a |
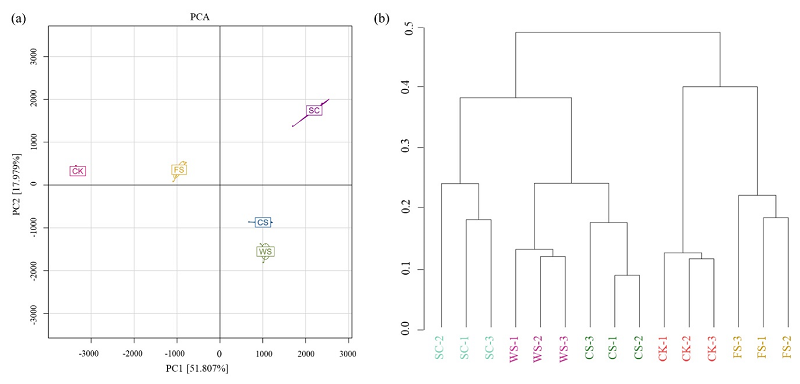
图1 不同种植方式土壤细菌群落的相似度分析(a)土壤细菌群落主成分分析(PCA图);(b)土壤细菌群落相似度树状图。CK:休耕;FS:休耕-大豆轮作; CS:玉米-大豆轮作;WS:小麦-大豆轮作;SC:大豆连作
Figure 1 Differences of soil bacterial community structure in different cropping systems(a) Principal component analysis of soil bacterial community (PCA diagram); (b) Similarity tree of soil bacterial community. CK: fallow; FS: fallow soybean rotation; CS: corn soybean rotation; WS: wheat soybean rotation; SC: soybean continuous cropping
| 多样性指数 Diversity index | CK | FS | CS | WS | SC |
|---|---|---|---|---|---|
| Chao1 | 4798±35b | 5541±205a | 4740±134b | 4592±49b | 4504±127b |
| ACE | 4803±27b | 5553±156a | 4659±179b | 4563±35b | 4509±106b |
| Shannon | 6.47±0.02b | 6.76±0.08a | 6.54±0.08b | 6.57±0.03b | 6.45±0.06b |
表2 不同种植方式土壤细菌群落的丰度及多样性
Table 2 Effects of different cropping systems on bacterial community abundance and diversity
| 多样性指数 Diversity index | CK | FS | CS | WS | SC |
|---|---|---|---|---|---|
| Chao1 | 4798±35b | 5541±205a | 4740±134b | 4592±49b | 4504±127b |
| ACE | 4803±27b | 5553±156a | 4659±179b | 4563±35b | 4509±106b |
| Shannon | 6.47±0.02b | 6.76±0.08a | 6.54±0.08b | 6.57±0.03b | 6.45±0.06b |
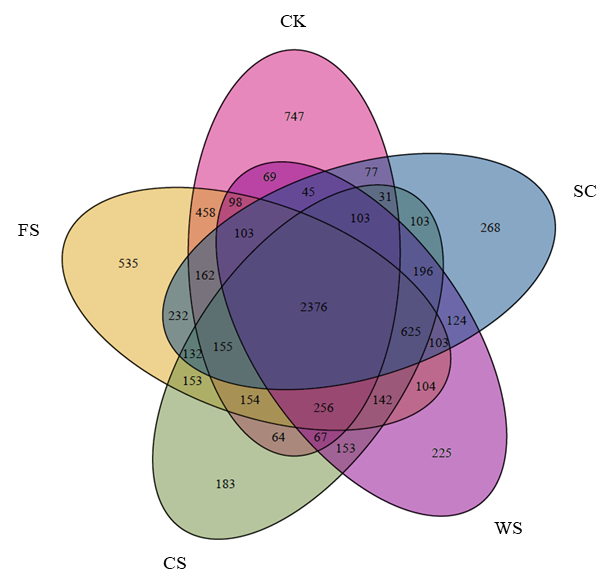
图2 不同种植方式土壤细菌OTUs的Venn图不同颜色代表不同种植方式,交叉区域代表共同拥有的物种。数字代表物种数
Figure 2 Venn diagram of soil bacteria OTUs under different cropping systems Different colors represent different cropping systems, and cross areas represent jointly owned species. Numbers represent the number of species
| 处理 Treatment | 潜在有益菌的相对丰度 Relative abundance of potentially beneficial bacteria | |||||
|---|---|---|---|---|---|---|
| 硝化螺旋菌 Nitrospira/% | 根瘤菌 Rhizobium/% | 芽孢杆菌 Bacillus/% | 厌氧蝇菌 Anaerolinea/%×10 | 固氮菌 Azotobacter/%×102 | 甲烷八叠球菌 Methanosarcina/%×102 | |
| CK | 0.32±0.01b | 0.05±0.00b | 0.01±0.00d | 0.00±0.06b | 0.00±0.00b | 0.00±0.00b |
| FS | 0.68±0.06a | 0.05±0.01b | 0.02±0.00d | 0.31±0.01a | 0.37±0.19a | 0.12±0.12a |
| CS | 0.34±0.01b | 0.22±0.02a | 0.07±0.00b | 0.01±0.01b | 0.00±0.00b | 0.00±0.00b |
| WS | 0.37±0.02b | 0.22±0.04a | 0.12±0.01a | 0.00±0.00b | 0.00±0.00b | 0.00±0.00b |
| SC | 0.21±0.02c | 0.32±0.06a | 0.05±0.01c | 0.02±0.01b | 0.00±0.00b | 0.00±0.00b |
表3 不同种植方式下潜在有益菌的相对丰度
Table 3 Relative abundance of potentially beneficial bacteria in different cropping systems
| 处理 Treatment | 潜在有益菌的相对丰度 Relative abundance of potentially beneficial bacteria | |||||
|---|---|---|---|---|---|---|
| 硝化螺旋菌 Nitrospira/% | 根瘤菌 Rhizobium/% | 芽孢杆菌 Bacillus/% | 厌氧蝇菌 Anaerolinea/%×10 | 固氮菌 Azotobacter/%×102 | 甲烷八叠球菌 Methanosarcina/%×102 | |
| CK | 0.32±0.01b | 0.05±0.00b | 0.01±0.00d | 0.00±0.06b | 0.00±0.00b | 0.00±0.00b |
| FS | 0.68±0.06a | 0.05±0.01b | 0.02±0.00d | 0.31±0.01a | 0.37±0.19a | 0.12±0.12a |
| CS | 0.34±0.01b | 0.22±0.02a | 0.07±0.00b | 0.01±0.01b | 0.00±0.00b | 0.00±0.00b |
| WS | 0.37±0.02b | 0.22±0.04a | 0.12±0.01a | 0.00±0.00b | 0.00±0.00b | 0.00±0.00b |
| SC | 0.21±0.02c | 0.32±0.06a | 0.05±0.01c | 0.02±0.01b | 0.00±0.00b | 0.00±0.00b |
| 菌门 Phylum | 有机质 OM | 有效N Available N | 有效P Available P | 有效K Available K | pH | 有效Cu Available Cu | 有效Zn Available Zn | 有效Mn Available Mn | 有效Fe Available Fe |
|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria | -0.59* | 0.67** | 0.82** | ||||||
| Acidobacteria | 0.64* | 0.55* | -0.72** | -0.81** | |||||
| Bacteroidetes | 0.60* | 0.52* | 0.60* | 0.64* | |||||
| Actinobacteria | -0.66** | -0.61* | -0.70** |
表4 土壤细菌优势菌门与土壤化学性质相关关系
Table 4 Correlations between dominant phyla of soil bacteria and soil chemical properties
| 菌门 Phylum | 有机质 OM | 有效N Available N | 有效P Available P | 有效K Available K | pH | 有效Cu Available Cu | 有效Zn Available Zn | 有效Mn Available Mn | 有效Fe Available Fe |
|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria | -0.59* | 0.67** | 0.82** | ||||||
| Acidobacteria | 0.64* | 0.55* | -0.72** | -0.81** | |||||
| Bacteroidetes | 0.60* | 0.52* | 0.60* | 0.64* | |||||
| Actinobacteria | -0.66** | -0.61* | -0.70** |
| 菌属 Genus | 菌门 Phylum | N | P | K | OM | pH | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gp4 | Acidobacteria | 0.52* | -0.66** | -0.68** | -0.55* | ||||||
| Gp6 | Acidobacteria | 0.65** | -0.67** | 0.77** | -0.76** | 0.56* | |||||
| Gemmatimonas | Gemmatimonadetes | -0.71** | -0.70** | ||||||||
| Gp1 | Acidobacteria | -0.68** | 0.71** | -0.61* | |||||||
| Gaiella | Actinobacteria | -0.60* | -0.54* | ||||||||
| WPS-1 | candidate_division_WPS-1 | -0.63* | -0.53* | -0.55* | -0.70** | ||||||
| Flavobacterium | Bacteroidetes | 0.80** | 0.58* | 0.91** | |||||||
| Gp3 | Acidobacteria | 0.68** | |||||||||
| Rhizomicrobium | Proteobacteria | -0.71** | -0.71** | ||||||||
| Rhodanobacter | Proteobacteria | -0.58* | 0.55* | -0.58* | -0.74** | 0.60* | -0.61 | ||||
| Terrimonas | Bacteroidetes | 0.76** | -0.52* | 0.66** | |||||||
| Opitutus | Verrucomicrobia | 0.52 | 0.76** | 0.54* | |||||||
| Pedobacter | Bacteroidetes | -0.71** | 0.69** | -0.64** | 0.77** |
表5 土壤细菌优势菌属与土壤化学性质相关关系
Table 5 Correlations between potentially beneficial bacteria and soil chemical properties
| 菌属 Genus | 菌门 Phylum | N | P | K | OM | pH | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gp4 | Acidobacteria | 0.52* | -0.66** | -0.68** | -0.55* | ||||||
| Gp6 | Acidobacteria | 0.65** | -0.67** | 0.77** | -0.76** | 0.56* | |||||
| Gemmatimonas | Gemmatimonadetes | -0.71** | -0.70** | ||||||||
| Gp1 | Acidobacteria | -0.68** | 0.71** | -0.61* | |||||||
| Gaiella | Actinobacteria | -0.60* | -0.54* | ||||||||
| WPS-1 | candidate_division_WPS-1 | -0.63* | -0.53* | -0.55* | -0.70** | ||||||
| Flavobacterium | Bacteroidetes | 0.80** | 0.58* | 0.91** | |||||||
| Gp3 | Acidobacteria | 0.68** | |||||||||
| Rhizomicrobium | Proteobacteria | -0.71** | -0.71** | ||||||||
| Rhodanobacter | Proteobacteria | -0.58* | 0.55* | -0.58* | -0.74** | 0.60* | -0.61 | ||||
| Terrimonas | Bacteroidetes | 0.76** | -0.52* | 0.66** | |||||||
| Opitutus | Verrucomicrobia | 0.52 | 0.76** | 0.54* | |||||||
| Pedobacter | Bacteroidetes | -0.71** | 0.69** | -0.64** | 0.77** |
| 菌属 Genus | N | P | K | OM | pH | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrospira | 0.78** | 0.53* | 0.52* | 0.76** | ||||||
| Rhizobium | -0.56* | -0.71** | -0.75** | -0.79** | ||||||
| Bacillu | -0.59* | -0.52* | -0.84** | |||||||
| Anaerolinea | 0.60* | 0.53* | 0.68** | 0.73** | ||||||
| Azotobacter | 0.57* | 0.57* | 0.61* | |||||||
| Methanosarcina | 0.64* | 0.52* | 0.57* |
表6 潜在有益菌与土壤化学性质的相关性
Table 6 Correlations between potentially beneficial bacteria and soil chemical properties
| 菌属 Genus | N | P | K | OM | pH | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrospira | 0.78** | 0.53* | 0.52* | 0.76** | ||||||
| Rhizobium | -0.56* | -0.71** | -0.75** | -0.79** | ||||||
| Bacillu | -0.59* | -0.52* | -0.84** | |||||||
| Anaerolinea | 0.60* | 0.53* | 0.68** | 0.73** | ||||||
| Azotobacter | 0.57* | 0.57* | 0.61* | |||||||
| Methanosarcina | 0.64* | 0.52* | 0.57* |
| [1] |
AI C, LIANG G Q, SUN J W, et al., 2015. Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils[J]. Soil Biology and Biochemistry, 80: 70-78.
DOI URL |
| [2] |
BENITEZ M S, OSBORNE S L, LEHMAN R M, 2017. Previous crop and rotation history effects on maize seedling health and associated rhizosphere microbiome[J]. Scientific Reports, 7(1): 15709.
DOI URL |
| [3] |
EDGAR R C, 2013. UPARSE: Highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 10(10): 996-998.
DOI URL |
| [4] |
FAN F L, ZHANG F S, LU Y H, 2011. Linking plant identity and interspecific competition to soil nitrogen cycling through ammonia oxidizer communities[J]. Soil Biology and Biochemistry, 43(1): 46-54.
DOI URL |
| [5] |
FIERER N, LAUBERR C L, RAMIREZ K S, et al., 2012. Comparative metagenomics, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients[J]. The ISME journal, 6(5): 1007-1017.
DOI URL |
| [6] |
HAMID M I, HUSSAIN M, WU Y P, et al., 2017. Successive soybean-monoculture cropping assembles rhizosphere microbial communities for the soil suppression of soybean cyst nematode[J]. FEMS Microbiol Ecology, 93(1): fiw222.
DOI URL |
| [7] |
LI J, WEN Y, LI X, et al., 2018. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain[J]. Soil and Tillage Research, 175: 281-290.
DOI URL |
| [8] |
LI X G, DING C F, ZHANG T L, et al., 2014. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing[J]. Soil Biology and Biochemistry, 72: 11-18.
DOI URL |
| [9] |
LI X Z, RUI J P, MAO Y J, et al., 2014. Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar[J]. Soil Biology and Biochemistry, 68: 392-401.
DOI URL |
| [10] |
LIU H, PAN F J, HAN X Z, et al., 2019. Response of soil fungal community structure to long-term continuous soybean cropping[J]. Frontiers in Microbiology, 9: 3316.
DOI URL |
| [11] |
LIU J J, SUI Y Y, YU Z H, et al., 2014. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China[J]. Soil Biology and Biochemistry, 70: 113-122.
DOI URL |
| [12] |
LIU J J, YU Z H, YAO Q, et al., 2017. Distinct soil bacterial communities in response to the cropping system in a Mollisol of northeast China[J]. Applied Soil Ecology, 119: 407-416.
DOI URL |
| [13] |
MENDES R, KRUIJT M, DE BRUIJN I, et al., 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria[J]. Science, 332(6033): 1097-1100.
DOI URL |
| [14] |
MO A S, QIU Z Q, HE Q, et al., 2016. Effect of continuous monocropping of tomato on soil microorganism and microbial biomass carbon[J]. Communications in Soil Science and Plant Analysis, 47(9): 1069-1077.
DOI URL |
| [15] |
NAVARRO-NOYA Y E, GOMEZ-ACATA S, MONTOYA-CIRIACO N, et al., 2013. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem[J]. Soil Biology and Biochemistry, 65: 86-95.
DOI URL |
| [16] | OFEK-LALZAR M, SELA N, GOLDMAN-VORONOV M, et al., 2014. Niche and host-associated functional signatures of the root surface microbiome[J]. Nature Communications, 5(1): 1-9. |
| [17] |
PANG G, CAI F, LI R X, et al., 2017. Trichoderma-enriched organic fertilizer can mitigate microbiome degeneration of monocropped soil to maintain better plant growth[J]. Plant and Soil, 416(1-2): 181-192.
DOI URL |
| [18] |
PEREZ-BRANDAN C, ARZENO J L, HUIDOBRO J, et al., 2014. The effect of crop sequences on soil microbial, chemical and physical indicators and its relationship with soybean sudden death syndrome (complex of Fusarium species)[J]. Spanish Journal of Agricultural Research, 12(1): 252-264.
DOI URL |
| [19] |
PII Y, MIMMO T, TOMASI N, et al., 2015. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review[J]. Biology and Fertility of Soils, 51(4): 403-415.
DOI URL |
| [20] | SCHREINER K, HAGN A, KYSELKOVA M, et al., 2010. Comparison of barley succession and take-all disease as environmental factors shaping the rhizobacterial community during take-all decline[J]. American Society for Microbiology, 76(14): 4703-4712. |
| [21] |
SMITH R G, GROSS K L, ROBERTSON G P, 2008. Effects of crop diversity on agroecosystem function: Crop yield response[J]. Ecosystems, 11(3): 355-366.
DOI URL |
| [22] |
TAN Y, CUI Y S, LI H Y, et al., 2016. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices[J]. Microbiological Research, 194: 10-19.
DOI URL |
| [23] |
TIAN W, WANG L, LI Y, et al., 2015. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen[J]. Agriculture, Ecosystems & Environment, 213: 219-227.
DOI URL |
| [24] | VENTER O, SANDERSON E W, MAGRACH A, et al., 2016. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation[J]. Nature communications, 7(1): 1-11. |
| [25] |
WANG B B, LI R, RUAN Y Z, et al., 2015. Pineapple-banana rotation reduced the amount of Fusarium oxysporum more than maize-banana rotation mainly through modulating fungal communities[J]. Soil Biology and Biochemistry, 86: 77-86.
DOI URL |
| [26] |
WANG Y, TU C, CHENG L, et al., 2011. Long-term impact of farming practices on soil organic carbon and nitrogen pools and microbial biomass and activity[J]. Soil and Tillage Research, 117: 8-16.
DOI URL |
| [27] | XIONG W, ZHAO Q Y, XUE C, et al., 2016. Comparison of fungal community in black pepper-vanilla and vanilla monoculture systems associated with vanilla Fusarium wilt disease[J]. Frontiers in Microbiology, 7: 117. |
| [28] |
XUN W B, HUANG T, ZHAO J, et al., 2015. Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities[J]. Soil Biology and Biochemistry, 90: 10-18.
DOI URL |
| [29] |
YIN C T, JONES K L, PETERSON D E, et al., 2010. Members of soil bacterial communities sensitive to tillage and crop rotation[J]. Soil Biology and Biochemistry, 42(12): 2111-2118.
DOI URL |
| [30] |
ZENG Q C, AN S S, LIU Y, 2017. Soil bacterial community response to vegetation succession after fencing in the grassland of China[J]. Science of the Total Environment, 609: 2-10.
DOI URL |
| [31] | 蔡元锋, 吴宇澄, 王书伟, 等, 2014. 典型淹水稻田土壤微生物群落的基因转录活性及其主要生理代谢过程[J]. 微生物学报, 54(9): 1033-1044. |
| CAI Y F, WU Y C, WANG S W, et al., 2014. Microbial metabolism in typical flooded paddy soils[J]. Acta Microbiologica Sinica, 54(9): 1033-1044. | |
| [32] | 陈雪丽, 2015. 黑土区连作大豆根际微生物群落特征研究[D]. 北京: 中国科学院大学. |
| CHEN X L, 2015. Characterization of microorganism community in the rhizosphere of continuous cropping soybean in black soil[D]. Beijing: University of Chinese Academy of Sciences. | |
| [33] | 韩丽梅, 鞠会艳, 王树起, 等, 2000. 大豆连作微量元素营养障碍与调控效果研究[J]. 吉林农业大学学报, 22(1): 73-80. |
| HAN L M, JU H Y, WANG S Q, et al., 2000. The nutrtion barriers and regulating effects of micro element in continuous cropping soybean[J]. Journal of Jilin Agricultural University, 22(1): 73-80. | |
| [34] | 李锐, 刘瑜, 褚贵新, 2015. 不同种植方式对绿洲农田土壤酶活性与微生物多样性的影响[J]. 应用生态学报, 26(2): 490-496. |
| LI R, LIU Y, CHU G X, 2015. Effects of different cropping patterns on soil enzyme activities and soil microbial community diversity in oasis farmland[J]. Chinese Journal of Applied Ecology, 26(2): 490-496. | |
| [35] | 李玉洁, 王慧, 赵建宁, 等, 2015. 耕作方式对农田土壤理化因子和生物学特性的影响[J]. 应用生态学报, 26(3): 939-948. |
| LI Y J, WANG H, ZHAO J N, et al., 2015. Effects of tillage methods on soil physicochemical properties and biological characteristics in farmland: A review[J]. Chinese Journal of Applied Ecology, 26(3): 939-948. | |
| [36] | 刘株秀, 刘俊杰, 徐艳霞, 等, 2019. 不同大豆连作年限对黑土细菌群落结构的影响[J]. 生态学报, 39(12): 4337-4346. |
| LIU Z X, LIU J J, XU Y X, et al., 2019. Effects of continuous cropping years of soybean on the bacterial community structure in black soil[J]. Acta Ecologica Sinica, 39(12): 4337-4346. | |
| [37] | 鲁如坤, 2000. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社: 10-150. |
| LU R K, 2000. Soil agrochemical analysis methods[M]. Beijing: China Agricultural Science and Technology Press: 10-150. | |
| [38] | 潘孝晨, 唐海明, 肖小平, 等, 2019. 不同耕作和秸秆还田模式对紫云英-双季稻土壤微生物生物量碳、氮含量的影响[J]. 生态环境学报, 28(8): 1585-1595. |
| PAN X C, TANG H M, XIAO X P, et al., 2019. Effects of different soil tillage and returning crop residues systems on soil microbial biomass carbon and nitrogen under Chinese milk vetch and double-cropping rice field[J]. Ecology and Environmental Sciences, 28(8): 1585-1595. | |
| [39] | 沈冰洁, 祝贞科, 袁红朝, 等, 2015. 不同种植方式对亚热带红壤微生物多样性的影响[J]. 环境科学, 36(10): 3839-3844. |
| SHEN B J, ZHU Z K, YUAN H C, et al., 2015. Effects of Different Plantation Type on the Abundance and Diversity of Soil Microbes in Subtropical Red Soils[J]. Environmental Science, 36(10): 3839-3844. | |
| [40] | 宋杰, 2016. 连作土壤寄生真菌多样性及对大豆胞囊线虫抑制作用[D]. 哈尔滨: 东北农业大学. |
| SONG J, 2016. Diversity and suppressive effect of parasitic fungi on soybean cyst nematode in soybean monoculture soil[D]. Harbin: Northeast Agricultural University. | |
| [41] | 宋长青, 吴金水, 陆雅海, 等, 2013. 中国土壤微生物学研究10年回顾[J]. 地球科学进展, 28(10): 1087-1105. |
| SONG C Q, WU J S, LU Y H, et al., 2013. Advances of soil microbiology in the last decade in China[J]. Advances in Earth Science, 28(10): 1087-1105. | |
| [42] | 王闯进, 2014. 大豆连作对根际土壤生物群落的影响[D]. 北京: 中国农业大学. |
| WANG C J, 2014. The impact of continuous soybean monoculture on soil communities in the rhizosphere[D]. Beijing: China Agricultural University. | |
| [43] | 王芳, 陈井生, 刘大伟, 2018. 不同种植方式大豆根际土壤细菌多样性分析[J]. 作物学报, 44(10): 1539-1547. |
|
WANG F, CHEN J S, LIU D W, 2018. Bacterial Diversity of Soybean Rhizosphere Soil under Different Cropping Patterns[J]. Acta Agronomica Sinica, 44(10): 1539-1547.
DOI URL |
|
| [44] | 夏围围, 贾仲君, 2014. 高通量测序和DGGE分析土壤微生物群落的技术评价[J]. 微生物学报, 54(12): 1489-1499. |
| XIA W W, JIA Z J, 2014. Comparative analysis of soil microbial communities by pyrosequencing and DGGE[J]. Acta Microbiologica Sinica, 54(12): 1489-1499. | |
| [45] | 徐光辉, 王洋, 王继红, 等, 2018. 休耕轮作对农田土壤微生物量碳的影响[J]. 土壤通报, 49(4): 897-901. |
| XU G H, WANG Y, WANG J H, et al., 2018. Effect of Fallow Rotation on Microbial Biomass Carbon in Farmland[J]. Chinese Journal of Soil Science, 49(4): 897-901. | |
| [46] |
周岚, 杨永, 王占海, 等, 2013. 玉米-大豆轮作及氮肥施用对土壤细菌群落结构的影响[J]. 作物学报, 39(11): 2016-2022.
DOI |
|
ZHOU L, YANG Y, WANG Z H, et al., 2013. Influence of Maize-Soybean Rotation and N Fertilizer on Bacterial Community Composition[J]. Acta Agronomica Sinica, 39(11): 2016-2022.
DOI |
|
| [47] | 朱琳, 曾椿淋, 李雨青, 等, 2017. 基于高通量测序的大豆连作土壤细菌群落多样性分析[J]. 大豆科学, 36(3): 419-424. |
| ZHU L, ZENG C L, LI Y Q, et al., 2017. The characteristic of bacterial community diversity in soybean field with continuous cropping based on the high-throughput sequencing[J]. Soybean Science, 36(3): 419-424. | |
| [48] | 朱英波, 史凤玉, 张瑞敬, 等, 2014. 黑龙江大豆轮作和连作土壤细菌群落多样性比较[J]. 植物保护学报, 41(4): 403-409. |
| ZHU Y B, SHI F Y, ZHANG R J, et al., 2014. Comparison of bacterial diversity in rotational and continuous soybean cropping soils in Heilongjiang[J]. Journal of Plant Protection, 41(4): 403-409. |
| [1] | 陈俊芳, 吴宪, 刘啸林, 刘娟, 杨佳绒, 刘宇. 不同土壤水分下元素化学计量对微生物多样性的塑造特征[J]. 生态环境学报, 2023, 32(5): 898-909. |
| [2] | 王洁, 单燕, 马兰, 宋延静, 王向誉. 秸秆/生物质炭协同还田措施对黄河三角洲盐碱土壤的改良效果研究[J]. 生态环境学报, 2023, 32(1): 90-98. |
| [3] | 王礼霄, 刘晋仙, 柴宝峰. 华北亚高山土壤细菌群落及氮循环对退耕还草的响应[J]. 生态环境学报, 2022, 31(8): 1537-1546. |
| [4] | 花莉, 成涛之, 梁智勇. 固定化混合菌对陕北黄土地区石油污染土壤的修复效果[J]. 生态环境学报, 2022, 31(8): 1610-1615. |
| [5] | 朱奕豪, 李青梅, 刘晓丽, 李娜, 宋凤玲, 陈为峰. 不同土地整治类型新增耕地土壤微生物群落特征研究[J]. 生态环境学报, 2022, 31(5): 909-917. |
| [6] | 王英成, 姚世庭, 金鑫, 俞文政, 芦光新, 王军邦. 三江源区高寒退化草甸土壤细菌多样性的对比研究[J]. 生态环境学报, 2022, 31(4): 695-703. |
| [7] | 刘红梅, 海香, 安克锐, 张海芳, 王慧, 张艳军, 王丽丽, 张贵龙, 杨殿林. 不同施肥措施对华北潮土区玉米田土壤固碳细菌群落结构多样性的影响[J]. 生态环境学报, 2022, 31(4): 715-722. |
| [8] | 邓晓, 武春媛, 杨桂生, 李怡, 李勤奋. 椰壳生物炭对海南滨海土壤的改良效果[J]. 生态环境学报, 2022, 31(4): 723-731. |
| [9] | 杨贤房, 陈朝, 郑林, 万智巍, 陈永林, 王远东. 稀土矿区不同土地利用类型土壤细菌群落特征及网络分析[J]. 生态环境学报, 2022, 31(4): 793-801. |
| [10] | 夏开, 邓鹏飞, 马锐豪, 王斐, 温正宇, 徐小牛. 马尾松次生林转换为湿地松和杉木林对土壤细菌群落结构和多样性的影响[J]. 生态环境学报, 2022, 31(3): 460-469. |
| [11] | 杨虎, 王佩瑶, 李小伟, 王继飞, 杨君珑. 贺兰山东坡不同植被类型的土壤真菌多样性及其群落结构[J]. 生态环境学报, 2022, 31(2): 239-247. |
| [12] | 张晓丽, 王国丽, 常芳弟, 张宏媛, 逄焕成, 张建丽, 王婧, 冀宏杰, 李玉义. 生物菌剂对根际盐碱土壤理化性质和微生物区系的影响[J]. 生态环境学报, 2022, 31(10): 1984-1992. |
| [13] | 刘秉儒. 土壤微生物呼吸热适应性与微生物群落及多样性对全球气候变化响应研究[J]. 生态环境学报, 2022, 31(1): 181-186. |
| [14] | 贾晨波, 郭洋, 马成莲, 苏建宇, 徐春燕. 宁杞1号枸杞健康株与根腐病患病株的土壤微生物群落和功能差异[J]. 生态环境学报, 2021, 30(9): 1831-1841. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
