生态环境学报 ›› 2025, Vol. 34 ›› Issue (7): 1121-1132.DOI: 10.16258/j.cnki.1674-5906.2025.07.012
贺环1,2( ), 周丹丹1,2,*(
), 周丹丹1,2,*( ), 马芷萱1,2, 李芳芳1,2, 秦珊珊1,2, 豆思娴1,2
), 马芷萱1,2, 李芳芳1,2, 秦珊珊1,2, 豆思娴1,2
收稿日期:2025-01-08
出版日期:2025-07-18
发布日期:2025-07-11
通讯作者:
*E-mail: 作者简介:贺环(1994年生),女,硕士研究生,主要研究方向为生物炭环境效应及污染物环境行为研究。E-mail: 1639477032@qq.com
基金资助:
HE Huan1,2( ), ZHOU Dandan1,2,*(
), ZHOU Dandan1,2,*( ), MA Zhixuan1,2, LI Fangfang1,2, QIN Shanshan1,2, DOU Sixian1,2
), MA Zhixuan1,2, LI Fangfang1,2, QIN Shanshan1,2, DOU Sixian1,2
Received:2025-01-08
Online:2025-07-18
Published:2025-07-11
摘要:
钙改性生物炭(CaBC)具有比表面积大、官能团种类丰富、矿质元素含量高等特性,对镉污染修复效果好。然而,生物炭施用过程中释放具有显著流动性的溶解性有机质(BDOM)会影响Cd(Ⅱ) 的环境行为。目前,关于钙改性生物炭中BDOM的释放特性及与其Cd(Ⅱ) 的结合特征尚不清晰。采用平行因子和二维相关光谱分析研究钙改性对生物炭BDOM特性的影响,其结果表明:1)钙改性使生物炭BDOM释放量显著增加,尤其是芳香性、疏水性及较大分子量组分;2)钙改性增加了类腐殖酸的相对分布并使其最先释放,但其抑制了类蛋白质的释放速度且类蛋白质含量和相对分布减少。BDOM与Cd(Ⅱ) 的络合实验结果表明:1)钙改性生物炭BDOM中类富里酸与Cd(Ⅱ) 结合速度最快,这与类富里酸相对分布最高有关;2)钙改性提高类蛋白质与Cd(Ⅱ) 的络合能力,但与Cd(Ⅱ) 的结合比例降低,这与改性后类蛋白质含量和相对分布较少有关;3)分子量和芳香性的增加促使BDOM与Cd(Ⅱ) 络合能力及形成的络合物稳定性增加。该研究有助于了解钙改性对BDOM与Cd(Ⅱ) 的结合特征的影响,并为钙改性生物炭在镉污染修复方面的应用提供参考依据。
中图分类号:
贺环, 周丹丹, 马芷萱, 李芳芳, 秦珊珊, 豆思娴. 钙改性对生物炭中溶解性有机质与Cd(Ⅱ)结合的影响[J]. 生态环境学报, 2025, 34(7): 1121-1132.
HE Huan, ZHOU Dandan, MA Zhixuan, LI Fangfang, QIN Shanshan, DOU Sixian. Effect of Calcium Modification on the Binding of Biochar-derived Dissolved Organic Matter with Cd(II)[J]. Ecology and Environmental Sciences, 2025, 34(7): 1121-1132.
| 生物炭 | 基本理化性质 | 元素组成/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 灰分质量分数/% | 比表面积/(m2·g−1) | 孔体积/(cm3·g−1) | C | H | O | N | H/C | O/C | (O+N)/C | ||
| CaBC | 6.80 | 21.07 | 4.36 | 0.012 | 53.08B | 4.28A | 19.95A | 1.37B | 0.97A | 0.28A | 0.30A | |
| BC | 7.55 | 21.02 | 3.08 | 0.010 | 57.84A | 4.23A | 16.67B | 1.71A | 0.88B | 0.22B | 0.24B | |
表1 生物炭基本理化性质及元素组成
Table 1 Basic physicochemical properties and elemental composition of biochar
| 生物炭 | 基本理化性质 | 元素组成/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 灰分质量分数/% | 比表面积/(m2·g−1) | 孔体积/(cm3·g−1) | C | H | O | N | H/C | O/C | (O+N)/C | ||
| CaBC | 6.80 | 21.07 | 4.36 | 0.012 | 53.08B | 4.28A | 19.95A | 1.37B | 0.97A | 0.28A | 0.30A | |
| BC | 7.55 | 21.02 | 3.08 | 0.010 | 57.84A | 4.23A | 16.67B | 1.71A | 0.88B | 0.22B | 0.24B | |
| 生物炭 | 准一级动力学 ln(Qe −Qt)=lnQe−K1t | 准二级动力学 t/Qt=1/K2 ×Qe2+t/Qe | Elovich Qt=a+blnt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg·g−1) | K1/(mg·g−1·h−1) | r2 | Qe/(mg·g−1) | K2/(mg·g−1·h−1) | r2 | a/(mg·g−1) | b/(mg·g−1·h−1) | r2 | |||
| CaBC | 10.54 | 0.88 | 0.791 | 11.04 | 0.13 | 0.957 | 6.86 | 1.13 | 0.981 | ||
| BC | 3.78 | 0.61 | 0.735 | 4.34 | 0.15 | 0.892 | 1.84 | 0.69 | 0.999 | ||
表2 BDOM释放动力学模型参数
Table 2 Parameters of BDOM release kinetics model
| 生物炭 | 准一级动力学 ln(Qe −Qt)=lnQe−K1t | 准二级动力学 t/Qt=1/K2 ×Qe2+t/Qe | Elovich Qt=a+blnt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg·g−1) | K1/(mg·g−1·h−1) | r2 | Qe/(mg·g−1) | K2/(mg·g−1·h−1) | r2 | a/(mg·g−1) | b/(mg·g−1·h−1) | r2 | |||
| CaBC | 10.54 | 0.88 | 0.791 | 11.04 | 0.13 | 0.957 | 6.86 | 1.13 | 0.981 | ||
| BC | 3.78 | 0.61 | 0.735 | 4.34 | 0.15 | 0.892 | 1.84 | 0.69 | 0.999 | ||
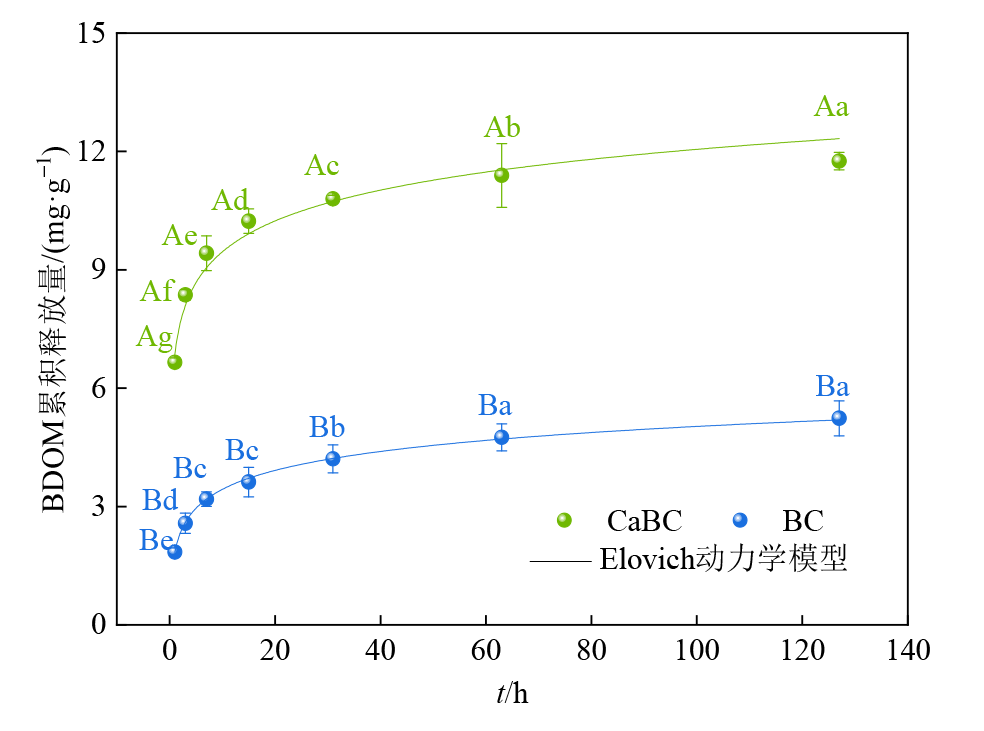
图2 BDOM累积释放含量动力学曲线 所有数值均为平均值±标准差(n=3);不同大写字母表示相同时间不同BDOM间差异显著;不同小写字母表示同一BDOM在不同释放时间之间差异显著(p<0.05);下同
Figure 2 Kinetic curve of cumulative release content of BDOM
| 生物炭 | 时间 | pH值 | 离子态钙质量浓度/ (mg·L−1) | 络合态钙质量浓度/ (mg·L−1) |
|---|---|---|---|---|
| CaBC | 1 | 6.00 | 160.90 | 175.79 |
| 127 | 7.59 | 61.54 | 15.73 | |
| BC | 1 | 6.73 | 6.27 | 2.13 |
| 127 | 8.15 | 3.80 | 2.67 |
表3 BDOM的pH值、离子态钙和络合态钙浓度
Table 3 BDOM pH value, ionic calcium and complex calcium concentration
| 生物炭 | 时间 | pH值 | 离子态钙质量浓度/ (mg·L−1) | 络合态钙质量浓度/ (mg·L−1) |
|---|---|---|---|---|
| CaBC | 1 | 6.00 | 160.90 | 175.79 |
| 127 | 7.59 | 61.54 | 15.73 | |
| BC | 1 | 6.73 | 6.27 | 2.13 |
| 127 | 8.15 | 3.80 | 2.67 |
| 生物炭 | t/h | BDOM荧光组分 | lgK | f/% | r2 |
|---|---|---|---|---|---|
| CaBC | 1 | C1 | 4.67 | 43.07 | 0.903 |
| C2 | 4.43 | 7.45 | 0.962 | ||
| C3 | 4.38 | 13.99 | 0.900 | ||
| 127 | C1 | 5.04 | 30.19 | 0.831 | |
| C2 | 4.45 | 2.93 | 0.886 | ||
| C3 | 4.63 | 5.82 | 0.816 | ||
| BC | 1 | C1 | 4.27 | 53.21 | 0.993 |
| C2 | 4.42 | 7.58 | 0.976 | ||
| C3 | 4.45 | 35.52 | 0.970 | ||
| 127 | C1 | 4.63 | 23.46 | 0.968 | |
| C2 | No | No | No | ||
| C3 | 4.62 | 5.95 | 0.974 |
表4 BDOM与Cd(Ⅱ)络合修正模型参数
Table 4 BDOM and Cd(Ⅱ) complexation modified model parameters
| 生物炭 | t/h | BDOM荧光组分 | lgK | f/% | r2 |
|---|---|---|---|---|---|
| CaBC | 1 | C1 | 4.67 | 43.07 | 0.903 |
| C2 | 4.43 | 7.45 | 0.962 | ||
| C3 | 4.38 | 13.99 | 0.900 | ||
| 127 | C1 | 5.04 | 30.19 | 0.831 | |
| C2 | 4.45 | 2.93 | 0.886 | ||
| C3 | 4.63 | 5.82 | 0.816 | ||
| BC | 1 | C1 | 4.27 | 53.21 | 0.993 |
| C2 | 4.42 | 7.58 | 0.976 | ||
| C3 | 4.45 | 35.52 | 0.970 | ||
| 127 | C1 | 4.63 | 23.46 | 0.968 | |
| C2 | No | No | No | ||
| C3 | 4.62 | 5.95 | 0.974 |
| [1] |
ALBRECHT R, LE PETIT J, TERROM G, et al., 2011. Comparison between UV spectroscopy and nirs to assess humification process during sewage sludge and green wastes co-composting[J]. Bioresource Technology, 102(6): 4495-4500.
DOI PMID |
| [2] | BAI H C, JIANG Z M, HE M J, et al., 2018. Relating Cd2+ binding by humic acids to molecular weight: A modeling and spectroscopic study[J]. Journal of Environmental Sciences, 70: 154-165. |
| [3] | BIAN R J, JOSEPH S, SHI W, et al., 2019. Biochar DOM for plant promotion but not residual biochar for metal immobilization depended on pyrolysis temperature[J]. Science of The Total Environment, 662: 571-580. |
| [4] | BRO R, 1997. PARAFAC. Tutorial and applications[J]. Chemometrics and Intelligent Laboratory Systems, 38(2): 149-171. |
| [5] | CHEN K, YANG Y M, ZHAO H, et al., 2023. Study on the cadmium and copper binding characteristics of dissolved organic matter released from human-feces-biochar (HFDOM) using parallel factor analysis (PARAFAC) and two-dimensional correlation spectroscopy (2D-COS)[J]. Environmental Science and Pollution Research, 30: 46900-46912. |
| [6] | CUI M, XU D Y, LIU X B, et al., 2024. Influence of spectral and molecular composition of dissolved organic matter on labile Cd mobility in riparian soils in the Three Gorges Reservoir, China[J]. Science of The Total Environment, 955: 176736. |
| [7] |
ELBISHLAWI H, JAFFE P R, 2015. Characterization of dissolved organic matter from a restored urban marsh and its role in the mobilization of trace metals[J]. Chemosphere, 127: 144-151.
DOI PMID |
| [8] | FENG D D, SUN H L, MA Y, et al., 2020. Catalytic mechanism of K and Ca on the volatile-biochar interaction for rapid pyrolysis of biomass: Experimental and simulation studies[J]. Energy & Fuels, 34: 9741-9753. |
| [9] | GAO Z Y, SHAN D X, HE J H, et al., 2023. Effects and mechanism on cadmium adsorption removal by CaCl2-modified biochar from selenium-rich straw[J]. Bioresource Technology, 370: 128563. |
| [10] | GUI X Y, LIU C, LI F Y, et al., 2020. Effect of pyrolysis temperature on the composition of DOM in manure-derived biochar[J]. Ecotoxicology and Environmental Safety, 197: 110597. |
| [11] | GUO X J, PENG Y Y, LI N X, et al., 2022. Effect of biochar-derived DOM on the interaction between Cu(II) and biochar prepared at different pyrolysis temperatures[J]. Journal of Hazardous Materials, 421: 126739. |
| [12] | HAMEED R, LI G L, SON Y H, et al., 2023. Structural characteristics of dissolved black carbon and its interactions with organic and inorganic contaminants: A critical review[J]. Science of The Total Environment, 872: 162210. |
| [13] | HE C J, HE X W, LI J J, et al., 2021. The spectral characteristics of biochar-derived dissolved organic matter at different pyrolysis temperatures[J]. Journal of Environmental Chemical Engineering, 9(5): 106075. |
| [14] | HE C J, HE X W, YUAN R, et al., 2022. Binding characteristics of Pb and Zn to low-temperature feces-based biochar-derived DOM revealed by EEM-PARAFAC combined with general and moving-window two-dimensional correlation analyses[J]. Environmental Science and Pollution Research, 30: 27525-27538. |
| [15] |
HE W, HUR J, 2015. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter[J]. Water Research, 83: 217-226.
DOI PMID |
| [16] | HUANG M, LI Z W, CHEN M, et al., 2020. Dissolved organic matter released from rice straw and straw biochar: Contrasting molecular composition and lead binding behaviors[J]. Science of The Total Environment, 739: 140378. |
| [17] | HUANG M, LI Z W, LUO N L, et al., 2019. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals[J]. Science of The Total Environment, 646: 220-228. |
| [18] | HUANG M, LIAO Z, LI Z W, et al., 2022. Effects of pyrolysis temperature on proton and cadmium binding properties onto biochar-derived dissolved organic matter: Roles of fluorophore and chromophore[J]. Chemosphere, 299: 134313. |
| [19] | JI Y N, ZHENG N, AN Q R, et al., 2024. Enhanced immobilization of cadmium and lead in contaminated soil using calcium alginate-modified HAP biochar: Improvements in soil health and microbial diversity[J]. Environmental Pollution, 357: 124445. |
| [20] | JIANG S J, DAI G L, RASHID M S, et al., 2024. Effects of BC on metal uptake by crops (availability) and the vertical migration behavior in soil: A 3-year field experiments of crop rotation[J]. Chemosphere, 350: 141075. |
| [21] | JIN C S, LI Z W, HURSTHOUSE A S, et al., 2023. Manganese oxides mediated dissolve organic matter compositional changes in lake sediment and cadmium binding characteristics[J]. Ecotoxicology and Environmental Safety, 256: 114916. |
| [22] | LENG E W, WANG Y, GONG X, et al., 2017. Effect of KCl and CaCl2 loading on the formation of reaction intermediates during cellulose fast pyrolysis[J]. Proceedings of the Combustion Institute, 36(2): 2263-2270. |
| [23] | LI D Q, LI C, FAN M J, et al., 2023a. Investigation of property of biochar in staged pyrolysis of cellulose[J]. Journal of Analytical and Applied Pyrolysis, 172: 105999. |
| [24] | LI D M, WANG Z Y, YANG Y X, et al., 2023b. Characterization of copper binding to different molecular weight fractions of dissolved organic matter in surface water[J]. Journal of Environmental Management, 341: 118067. |
| [25] |
LI G, KHAN S, IBRAHIM M, et al., 2018. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium[J]. Journal of Hazardous Materials, 348: 100-108.
DOI PMID |
| [26] |
LI H B, DONG X L, DA SILVA E B, et al., 2017. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications[J]. Chemosphere, 178: 466-478.
DOI PMID |
| [27] | LI L P, LIU Y H, REN D, et al., 2022. Characteristics and chlorine reactivity of biochar-derived dissolved organic matter: Effects of feedstock type and pyrolysis temperature[J]. Water Research, 211: 118044. |
| [28] | LIANG T, ZHOU G P, CHANG D N, et al., 2024. The dissolved organic matter from the co-decomposition of Chinese milk vetch and rice straw induces the strengthening of Cd remediation by Fe-modified biochar[J]. Biochar, 6: 27. |
| [29] | LIU C H, CHU W Y, LI H, et al., 2019. Quantification and characterization of dissolved organic carbon from biochars[J]. Geoderma, 335: 161-169. |
| [30] | LIU J B, YANG W T, ZHOU H, et al., 2024a. Exploring the mechanisms of organic fertilizers on Cd bioavailability in rice fields: Environmental behavior and effect factors[J]. Ecotoxicology and Environmental Safety, 285: 117094. |
| [31] | LIU X R, WEI L H, JIANG J Y, et al., 2024b. New insights into the effect of pyrolysis temperature on the spectroscopy properties of dissolved organic matter in manure-based biochar[J]. Environmental Science and Pollution Research, 31: 18527-18539. |
| [32] | LIU Y C, GAO Z L, JI X G, et al., 2023. Efficient Adsorption of tebuconazole in aqueous solution by calcium modified water hyacinth-based biochar: Adsorption kinetics, mechanism, and feasibility[J]. Molecules, 28(8): 3478. |
| [33] | MURPHY K R, BUTLER K D, SPENCER R G M, et al., 2010. Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison[J]. Environmental Science & Technology, 44(24): 9405-9412. |
| [34] | NAN H Y, YIN J X, YANG F, et al., 2021. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration[J]. Environmental Pollution, 287: 117566. |
| [35] | NODA I, 2012. Close-up view on the inner workings of two-dimensional correlation spectroscopy[J]. Vibrational Spectroscopy, 60: 146-153. |
| [36] | OU Q, XU Y H, HE Q, et al., 2021. Deposition behavior of dissolved black carbon on representative surfaces: Role of molecular conformation[J]. Journal of Environmental Chemical Engineering, 9(5): 105921. |
| [37] | REN N N, TANG Y Y, LI M, 2018. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar[J]. Process Safety and Environmental Protection, 115: 70-78. |
| [38] | STEDMON C A, BRO R, 2008. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial[J]. Limnology and Oceanography: Methods, 6(11): 572-579. |
| [39] | SUN D Z, LI F Y, JIN J W, et al., 2022. Qualitative and quantitative investigation on adsorption mechanisms of Cd(II) on modified biochar derived from co-pyrolysis of straw and sodium phytate[J]. Science of The Total Environment, 829: 154599. |
| [40] | WANG C C, ZHANG Q C, YAN C A, et al., 2023a. Heavy metal(loid)s in agriculture soils, rice, and wheat across China: Status assessment and spatiotemporal analysis[J]. Science of The Total Environment, 882: 163361. |
| [41] | WANG J B, KANG Y X, DUAN H T, et al., 2022. Remediation of Cd2+ in aqueous systems by alkali-modified (Ca) biochar and quantitative analysis of its mechanism[J]. Arabian Journal of Chemistry, 15: 103750. |
| [42] | WANG S Y, WANG Y J, WANG X Y, et al., 2024. Study on adsorption of Cd in solution and soil by modified biochar-calcium alginate hydrogel[J]. Gels, 10(6): 388. |
| [43] | WANG X S, YU G, LIN H, et al., 2020. Advances in bioremediation of cadmium-contaminated soils[J]. IOP Conference Series: Earth and Environmental Science, 508: 012006. |
| [44] | WANG Y F, LI J E, XU L, et al., 2023b. The effect and spectral response mechanism of dissolved organic matter (DOM) in Pb(II) adsorption onto biochar[J]. Journal of Environmental Chemical Engineering, 11(5): 111115. |
| [45] |
WU J, ZHANG H, SHAO L M, et al., 2012. Fluorescent characteristics and metal binding properties of individual molecular weight fractions in municipal solid waste leachate[J]. Environmental Pollution, 162: 63-71.
DOI PMID |
| [46] | WU W Z, YAN B B, ZHONG L, et al., 2021. Combustion ash addition promotes the production of K-enriched biochar and K release characteristics[J]. Journal of Cleaner Production, 311: 127557. |
| [47] |
XIAO R, WANG J J, GASTON L A, et al., 2018. Biochar produced from mineral salt-impregnated chicken manure: Fertility properties and potential for carbon sequestration[J]. Waste Management, 78: 802-810.
DOI PMID |
| [48] | XIAO Y, WIESNER M R, 2013. Transport and retention of selected engineered nanoparticles by porous media in the presence of a biofilm[J]. Environmental Science & Technology, 47(5): 2246-2253. |
| [49] | XIU L Q, GU W Q, SUN Y Y, et al., 2023. The fate and supply capacity of potassium in biochar used in agriculture[J]. Science of The Total Environment, 902: 165969. |
| [50] |
XU Y G, QI F J, YAN Y B, et al., 2023. The interaction of different chlorine-based additives with swine manure during pyrolysis: Effects on biochar properties and heavy metal volatilization[J]. Waste Management, 169: 52-61.
DOI PMID |
| [51] | YU S H, ZHANG H Y, NI J Z, et al., 2023. Spectral characteristics coupled with self-organizing maps analysis on different molecular size-fractionated water-soluble organic carbon from biochar[J]. Science of The Total Environment, 857(Part 2): 159424. |
| [52] | ZEPP R G, SHELDON W M, MORAN M A, 2004. Dissolved organic fluorophores in southeastern US coastal waters: Correction method for eliminating Rayleigh and Raman scattering peaks in excitation-emission matrices[J]. Marine Chemistry, 89(1-4): 15-36. |
| [53] | ZHANG H Y, QIAN W, WU L, et al., 2022b. Spectral characteristics of dissolved organic carbon (DOC) derived from biomass pyrolysis: Biochar-derived DOC versus smoke-derived DOC, and their differences from natural DOC[J]. Chemosphere, 302: 134869. |
| [54] | ZHANG X Q, LI Y, YE J, et al., 2022a. The spectral characteristics and cadmium complexation of soil dissolved organic matter in a wide range of forest lands[J]. Environmental Pollution, 299: 118834. |
| [55] | ZHANG X Y, SU C, LIU X Y, et al., 2020. Periodical changes of dissolved organic matter (DOM) properties induced by biochar application and its impact on downward migration of heavy metals under flood conditions[J]. Journal of Cleaner Production, 275: 123787. |
| [56] | ZHAO C, WANG C C, LI J Q, et al., 2017. Interactions between copper(II) and DOM in the urban stormwater runoff: Modeling and characterizations[J]. Environmental Technology, 39(1): 120-129. |
| [57] | ZHOU L, ZHOU L, WU H B, et al., 2024. Effects of applying biochar on soil cadmium immobilisation and cadmium pollution control in lettuce (Lactuca sativa L.)[J]. Agriculture, 14(7): 1068. |
| [58] | ZHU Y, YI B J, HU H Y, et al., 2020. The relationship of structure and organic matter adsorption characteristics by magnetic cattle manure biochar prepared at different pyrolysis temperatures[J]. Journal of Environmental Chemical Engineering, 8(5): 104112. |
| [59] | 范行程, 葛俊杰, 谢越, 等, 2024. 蘑菇渣和稻秸堆肥中DOM与Cu2+的络合机制[J]. 生态与农村环境学报, 40(2): 285-292. |
| FAN X C, GE J J, XIE Y, et al., 2024. The binding properties of Cu(II) onto dissolved organic matter from mushroom residue and rice straw compost[J]. Journal of Ecology and Rural Environment, 40(2): 285-292. | |
| [60] |
刘玉灿, 高中鲁, 徐心怡, 等, 2024. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 43(8): 4630-4641.
DOI |
|
LIU Y C, GAO Z L, XU X Y, et al., 2024. Adsorption performance and mechanism of diuron from water by calcium-modified water hyacinth-based biochar[J]. Chemical Industry and Engineering Progress, 43(8): 4630-4641.
DOI |
|
| [61] | 袁冬海, 崔骏, 洪志强, 等, 2016. 白洋淀沉水植物腐解溶解性有机物与重金属的相互作用[J]. 环境工程学报, 10(5): 2184-2192. |
| YUAN D H, CUI J, HONG Z Q, et al., 2016. Interaction between dissolved organic matter released by macrophyte decomposition and heavy metal in Lake Baiyangdian[J]. Chinese Journal of Environmental Engineering, 10(5): 2184-2192. | |
| [62] | 张威宇, 李伟峻, 刘玉玲, 等, 2024. 人工湿地中沉积物溶解性有机质与Cd结合机制——以株洲市某人工湿地为例[J]. 农业环境科学学报, 43(6): 1377-1388. |
| ZHANG W Y, LI W J, LIU Y L, et al., 2024. Mechanism of binding between dissolved organic matter and cadmium in constructed wetland sediments: Taking a constructed wetland in Zhuzhou City as an example[J]. Journal of Agro-Environment Science, 43(6): 1377-1388. | |
| [63] | 赵雄威, 吴东明, 李勤奋, 等, 2022. 基于紫外-可见光光谱法研究长期不同施肥对砖红壤溶解性有机质化学性质的影响[J]. 光谱学与光谱分析, 42(10): 3210-3216. |
| ZHAO X W, WU D M, LI Q F, et al., 2022. Response of dissolved organie matter chemical properties to long term different fertilization in latosol: Insight from ultraviolet-visible spectroscopy[J]. Spectroscopy and Spectral Analysis, 42(10): 3210-3216. |
| [1] | 李雪, 王震, 毛雪飞. 聚乙烯与聚丙烯微塑料对镉胁迫下水稻幼苗生长及抗氧化作用的影响[J]. 生态环境学报, 2025, 34(7): 1053-1063. |
| [2] | 崔雪丹, 段桂兰, 王向琴, 李志丰, 窦飞, 杜衍红, 袁雨珍, 刘传平, 李芳柏. 基于两地长期定位试验的铁改性木本泥炭修复中轻度镉砷污染稻田效果与土壤健康效应评价[J]. 生态环境学报, 2025, 34(4): 608-620. |
| [3] | 吴昕优, 涂晨, 刘国明, 杨帅, 王译, 王旭洋, 骆润来, 李忠元, 骆永明. 毫米级磁性复合黏土矿物修复材料的结构、性质及其对镉的吸附特征[J]. 生态环境学报, 2025, 34(4): 621-630. |
| [4] | 宁静, 王淳, 卢莞玲, 韦露. 斑马鱼暴露于镉和褪黑素引起肠道组织、氧化损伤及微生物多样性变化[J]. 生态环境学报, 2025, 34(1): 77-88. |
| [5] | 曹振宇, 涂晨, 刘颖, 韩军超, 邢倩雯, 骆永明. 趋磁细菌Magnetospirillum gryphiswaldense MSR-1对镉的生物吸附初步研究[J]. 生态环境学报, 2025, 34(1): 99-107. |
| [6] | 李林峰, 徐梓盛, 陈勇, 李奇, 林晓扬, 李义纯. 施硅水平对水稻根表铁膜和体内Cd累积分布的影响[J]. 生态环境学报, 2024, 33(5): 781-790. |
| [7] | 王室苹, 李梅, 安娅, 秦好丽. 镁改性增强小麦秸秆生物炭对镉的吸附能力:表面络合模型研究[J]. 生态环境学报, 2024, 33(4): 617-625. |
| [8] | 张腾云, 王静, 高健磊, 葛文静, 王宗耀, 韩龙. 碱性农田土壤冬小麦不同生育期镉的迁移转化研究[J]. 生态环境学报, 2024, 33(3): 450-459. |
| [9] | 刘楚天, 郭栋栋, 侯磊, 梁启斌, 王艳霞, 施艳婷, 戚艳娥. 营养调控影响滇杨幼苗镉积累的效应模型分析[J]. 生态环境学报, 2024, 33(3): 460-468. |
| [10] | 官国庆, 黄紫琳, 江龙飞, 罗春玲. 伴矿景天对重金属-多环芳烃复合污染土壤有机污染物消减及微生物的影响[J]. 生态环境学报, 2024, 33(12): 1931-1943. |
| [11] | 纪晟莹, 李杰, 李鑫, 陶禹, 陈娟, 王晓玉. 环境与基因型互作对瓜类蔬菜镉积累的影响及产地土壤安全阈值研究[J]. 生态环境学报, 2024, 33(12): 1944-1952. |
| [12] | 范婉仪, 涂晨, 王顺扬, 吴昕优, 李烜桢, 骆永明. 不同品种烟草对轻度污染耕地土壤中镉的累积特征与减量修复潜力[J]. 生态环境学报, 2023, 32(8): 1516-1524. |
| [13] | 王丽华, 王磊, 许端平, 薛杨. 煤胶体对重金属铜与镉的吸附特征研究[J]. 生态环境学报, 2023, 32(7): 1293-1300. |
| [14] | 李治梅, 安娅, 李梅, 王室苹, 秦好丽. 巯基/铁基功能化蒙脱土对土壤镉的钝化行为研究[J]. 生态环境学报, 2023, 32(7): 1301-1312. |
| [15] | 李振国, 郝星雨, 贺甜莲, 景蕊, 荣成, 顾承真, 郑新宇. 竹醋液对紫苏镉毒的缓解效应研究[J]. 生态环境学报, 2023, 32(7): 1313-1324. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
