生态环境学报 ›› 2024, Vol. 33 ›› Issue (2): 272-281.DOI: 10.16258/j.cnki.1674-5906.2024.02.011
蓝浚1,2( ), 陈冠虹2,*(
), 陈冠虹2,*( ), 张俊涛3, Hemmat-Jou Mohammad Hossein2, 舒小华1, 方利平2, 李芳柏2
), 张俊涛3, Hemmat-Jou Mohammad Hossein2, 舒小华1, 方利平2, 李芳柏2
收稿日期:2023-12-03
出版日期:2024-02-18
发布日期:2024-04-03
通讯作者:
*陈冠虹。E-mail: ghchen@soil.gd.cn作者简介:蓝浚(2000年生),男,硕士研究生,研究方向为土壤锑污染控制。E-mail: lanjun9527@163.com
基金资助:
LAN Jun1,2( ), CHEN Guanhong2,*(
), CHEN Guanhong2,*( ), ZHANG Juntao3, HEMMAT-JOU Mohammad Hossein2, SHU Xiaohua1, FANG Liping2, LI Fangbai2
), ZHANG Juntao3, HEMMAT-JOU Mohammad Hossein2, SHU Xiaohua1, FANG Liping2, LI Fangbai2
Received:2023-12-03
Online:2024-02-18
Published:2024-04-03
摘要:
微生物锑还原成矿有助于降低土壤锑的生物有效性和移动性,是土壤锑污染修复的重要策略之一。电子穿梭体AQDS能够加速土壤富集菌群锑还原速率,可能与其促进微生物呼吸及细胞生长有关。理解电子穿梭体(ES)介导微生物锑还原过程与机制可为土壤锑污染控制提供关键理论支撑。醌类和黄素类电子穿梭体(AQDS和FMN)存在时可能改变微生物呼吸代谢中的电子传递过程,然而ES介导下的微生物锑还原过程及转录响应机制尚不清楚。利用锑污染稻田土壤分离的兼性厌氧锑还原细菌Mesobacillus jeotgali PS1作为研究对象,探究醌类和黄素类电子穿梭体(AQDS和FMN)对菌株PS1锑还原过程及关键功能基因转录活性的影响。结果表明,菌株PS1驱动Sb(V)还原为Sb(III)过程中水溶态Sb(III) 随培养时间先累积后下降,培养72 h后水溶态锑去除率为64%,生成十四面体方锑矿,表明菌株PS1驱动锑还原成矿有助于锑的钝化。两种ES能够加速细菌锑还原反应,而对胞外生成的方锑矿晶型没有影响。通过定量分析菌株PS1潜在功能基因转录表达活性,结果表明AQDS相比FMN更能促进菌株PS1细胞膜二甲基亚砜还原酶(DMSOR)基因和胞内解毒型砷还原基因(arsC)转录活性,有助于增加菌株PS1呼吸代谢活性和胞内锑解毒从而加速锑还原,所以AQDS可能在强化微生物锑还原钝化中更具有优势。该研究揭示了不同电子穿梭体介导锑还原的微生物机制,为锑污染土壤生物修复提供重要理论支撑。
中图分类号:
蓝浚, 陈冠虹, 张俊涛, Hemmat-Jou Mohammad Hossein, 舒小华, 方利平, 李芳柏. 电子穿梭体介导土壤锑还原成矿的微生物机制[J]. 生态环境学报, 2024, 33(2): 272-281.
LAN Jun, CHEN Guanhong, ZHANG Juntao, HEMMAT-JOU Mohammad Hossein, SHU Xiaohua, FANG Liping, LI Fangbai. Microbial Mechanism of Electron Shuttle-mediated Antimony Reduction and Mineralization by Soil Microorganism[J]. Ecology and Environment, 2024, 33(2): 272-281.
| 目的基因 | 引物名称 | 引物序列 (5′-3′) | 扩增条件 |
|---|---|---|---|
| gyrB | gyrB-F | GGCGGTACACACGAATTTGG | 95 ℃预变性 2 min; 95 ℃ 10 s, 60 ℃ 20 s, 40个循环 |
| gyrB-R | CTTCACGCACGTCTTCTCCT | ||
| dmsB | dmsB-F | CCAGATCACGGATGAGGGCG | |
| dmsB-R | CGCGTCACTTGGGCAAACAG | ||
| nrfC | nrfC-F | GTAAGTGTCTGCCCGACCAA | |
| nrfC-R | TACCTTCTGCGACCAACTCG | ||
| nasA | nasA-F | CCTGCTACAACATGGGCAGA | |
| nasA-R | CTGGCCTTTACCGAGACGTT | ||
| fdnG | fdnG F | CATCCCACCAAATGACAGCC | |
| fdnG-R | GCAAGAACCGCTCCAAGAAC | ||
| arsC | arsC-F | AGGCGATGAATGAGGTGGGA | |
| arsC-R | GTTACCGGGCAGTGCTCATC |
表1 实时荧光定量PCR引物序列及反应条件
Table 1 Details of primer pairs and thermal cycling parameters for qPCR
| 目的基因 | 引物名称 | 引物序列 (5′-3′) | 扩增条件 |
|---|---|---|---|
| gyrB | gyrB-F | GGCGGTACACACGAATTTGG | 95 ℃预变性 2 min; 95 ℃ 10 s, 60 ℃ 20 s, 40个循环 |
| gyrB-R | CTTCACGCACGTCTTCTCCT | ||
| dmsB | dmsB-F | CCAGATCACGGATGAGGGCG | |
| dmsB-R | CGCGTCACTTGGGCAAACAG | ||
| nrfC | nrfC-F | GTAAGTGTCTGCCCGACCAA | |
| nrfC-R | TACCTTCTGCGACCAACTCG | ||
| nasA | nasA-F | CCTGCTACAACATGGGCAGA | |
| nasA-R | CTGGCCTTTACCGAGACGTT | ||
| fdnG | fdnG F | CATCCCACCAAATGACAGCC | |
| fdnG-R | GCAAGAACCGCTCCAAGAAC | ||
| arsC | arsC-F | AGGCGATGAATGAGGTGGGA | |
| arsC-R | GTTACCGGGCAGTGCTCATC |
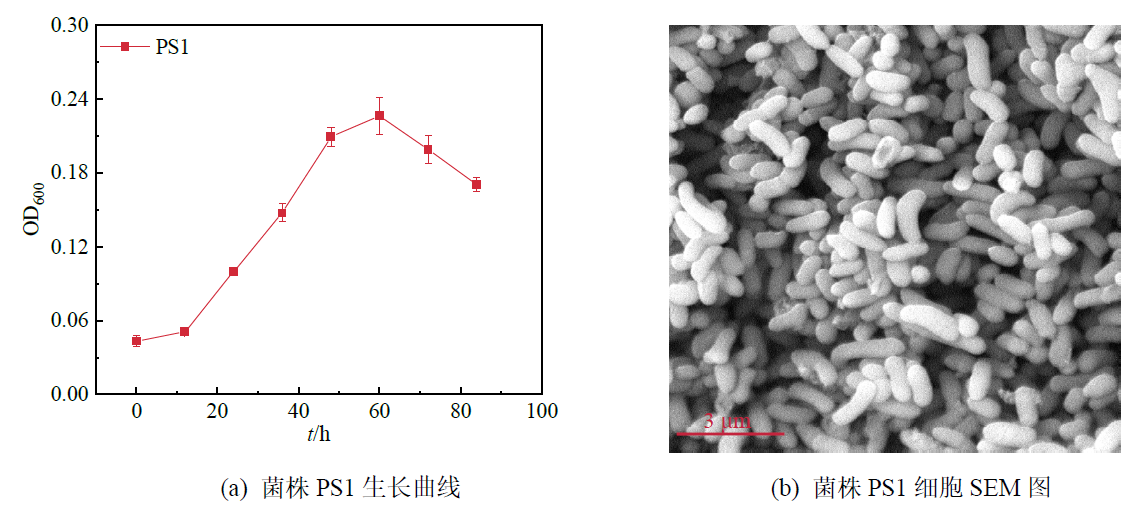
图1 乳酸和Sb(V)作为电子供体和受体条件下锑还原功能细菌PS1的生长曲线和细胞SEM图
Figure 1 Growth curve and SEM image of antimonate-reducing strain PS1 in the medium supplemented with lactate and Sb(V) as electron donor and acceptor
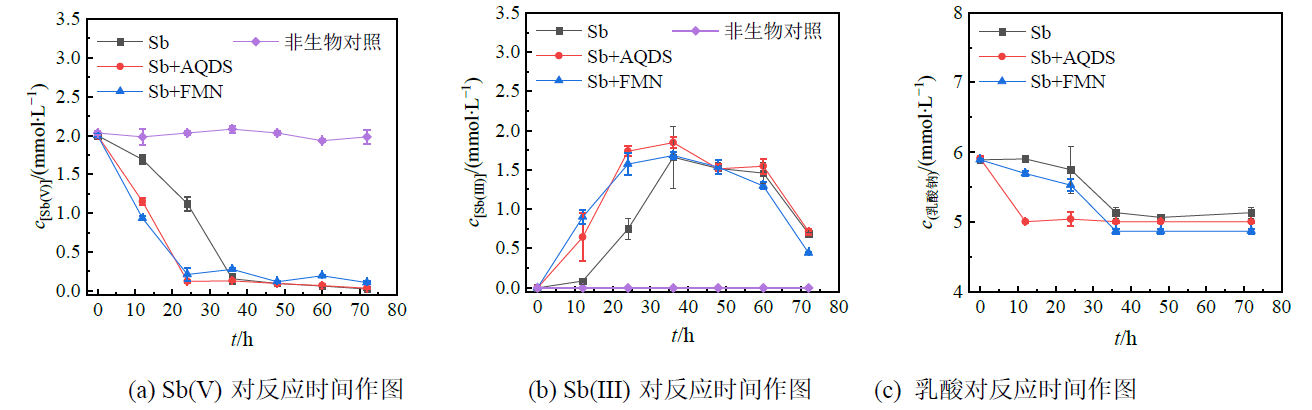
图2 电子穿梭体影响下菌株PS1培养72 h内Sb(V)、Sb(III)和乳酸随时间的浓度变化
Figure 2 Concentrations of Sb(V), Sb(III) and lactate over time in strain PS1 cultured for 72 h influenced by electron shuttles
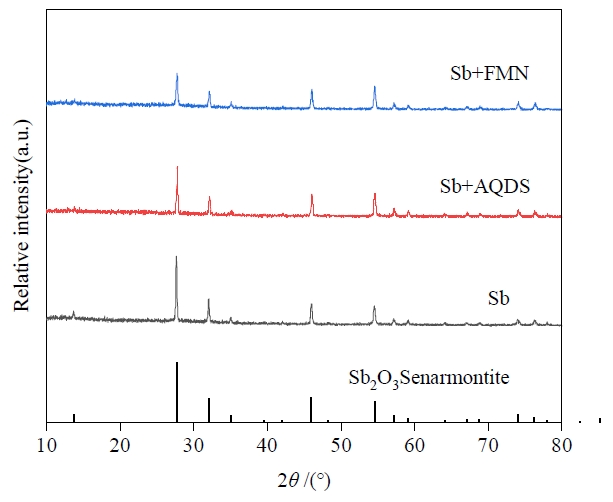
图3 Sb、Sb+AQDS和Sb+FMN处理中培养72 h后菌株PS1锑还原产物的XRD图谱
Figure 3 XRD patterns of antimony reduction products from strain PS1 after 72 h incubation in Sb, Sb+AQDS and Sb+FMN treatments
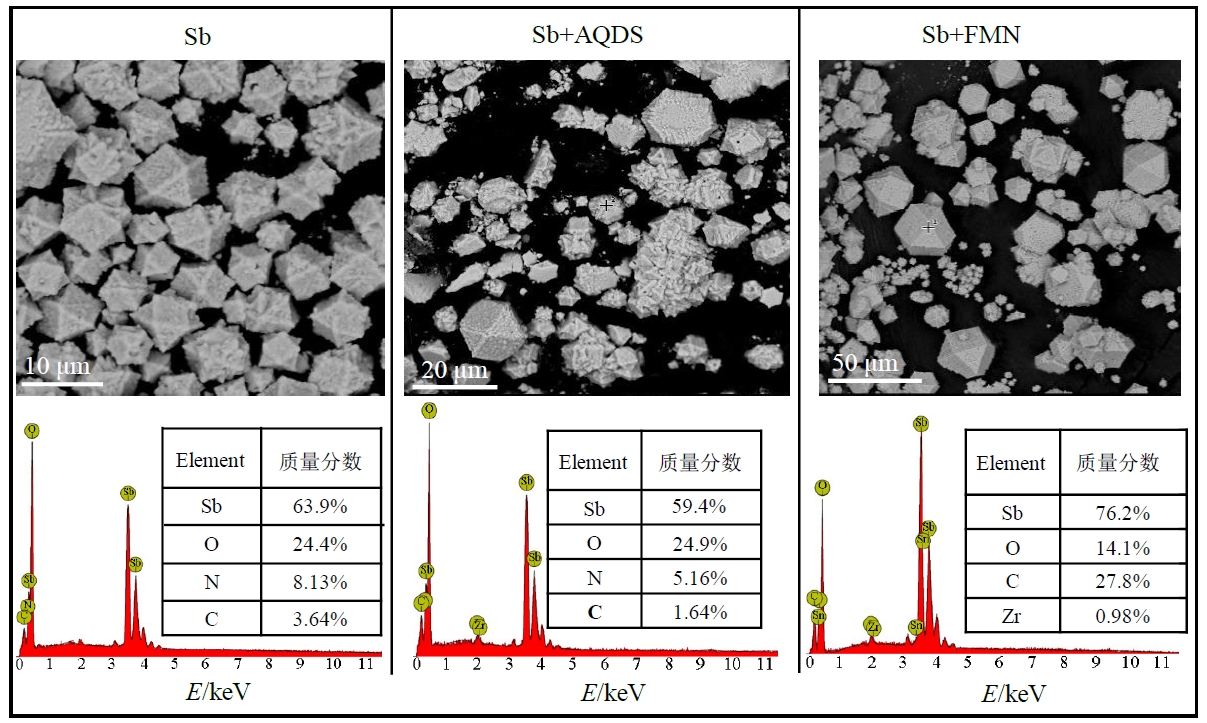
图4 Sb、Sb+AQDS和Sb+FMN处理中培养72 h后菌株PS1锑还原产物的SEM-EDS图
Figure 4 SEM-EDS plots of antimony reduction products of strain PS1 after 72 h incubation in Sb, Sb+AQDS and Sb+FMN treatments

图5 菌株PS1在不同处理中培养72 h后TEM-EDS图
Figure 5 TEM-EDS plots of cell-mineral precipitation of strain PS1 after 72 h incubation in Sb, Sb+AQDS and Sb+FMN treatment
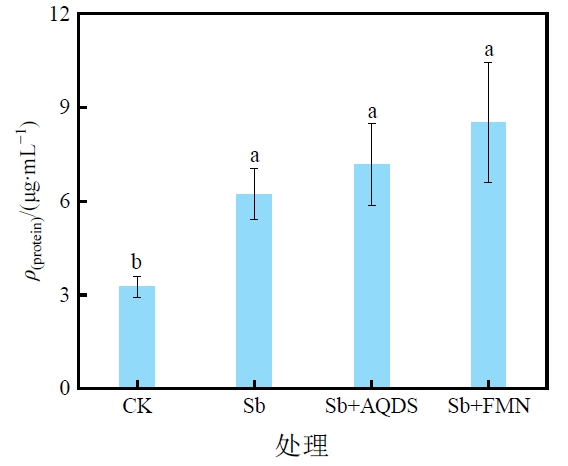
图6 菌株PS1在不同处理中培养72 h后的蛋白质浓度 不同小写字母代表处理之间具有显著性差异
Figure 6 Variability in protein amount of strain PS1 after 72 h incubation in CK, Sb, Sb+AQDS and Sb+FMN treatments
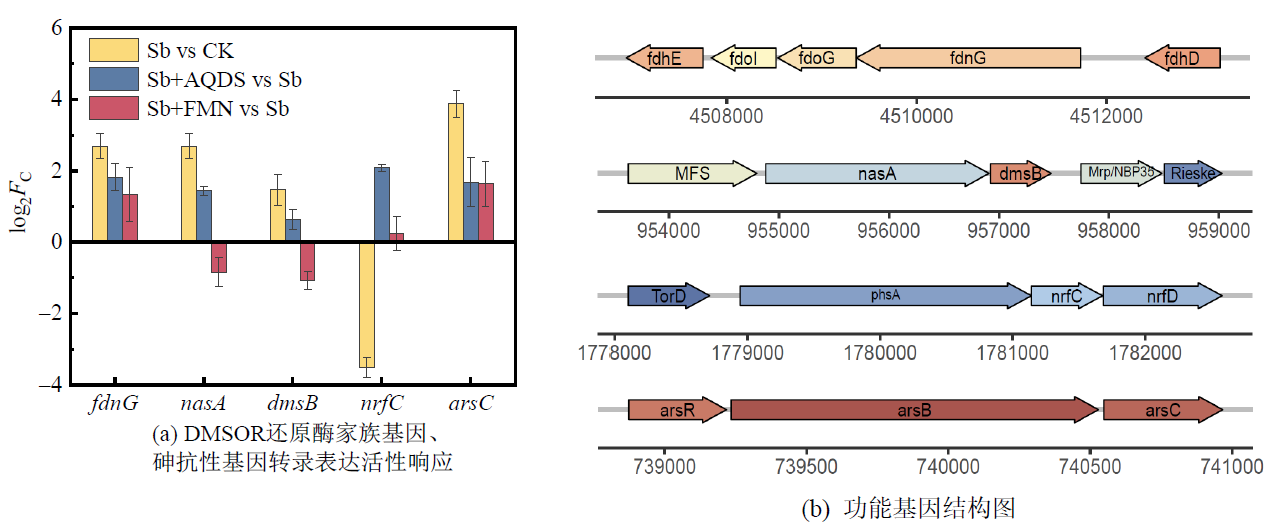
图7 不同处理下菌株PS1中DMSOR还原酶家族基因、砷抗性基因转录表达活性响应(Fc为差异倍数)
Figure 7 Transcriptional responses of DMSOR family genes and arsenic resistance genes in strain PS1 under different treatments (Fc is the variance multiplier)
| [1] |
ABIN C A, HOLLIBAUGH J T, 2013. Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism[J]. Environmental Science & Technology, 48(1): 681-688.
DOI URL |
| [2] |
ABIN C A, HOLLIBAUGH J T, 2019. Transcriptional response of the obligate anaerobe Desulfuribacillus stibiiarsenatis MLFW-2T to growth on antimonate and other terminal electron acceptors[J]. Environmental Microbiology, 21(2): 618-630.
DOI URL |
| [3] |
BOLAN N, KUMAR M, SINGH E, et al., 2022. Antimony contamination and its risk management in complex environmental settings: A review[J]. Environment International, 158: 106908.
DOI URL |
| [4] |
BUTCHER BRONWYN G, DEANE SHELLY M, RAWLINGS DOUGLAS E, 2000. The chromosomal arsenic resistance genes of thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli[J]. Applied and Environmental Microbiology, 66(5): 1826-1833.
DOI URL |
| [5] |
FILELLA M, BELZILE N, CHEN Y W, 2002. Antimony in the environment: a review focused on natural waters: II. Relevant solution chemistry[J]. Earth-Science Reviews, 59(1): 265-285.
DOI URL |
| [6] |
GUO X J, WU Z J, HE M C, et al., 2014. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure[J]. Journal of Hazardous Materials, 276: 339-345.
DOI PMID |
| [7] |
HARSHITHA R, ARUNRAJ D R, 2021. Real-time quantitative PCR: A tool for absolute and relative quantification[J]. Biochemistry and Molecular Biology Education, 49(5): 800-812.
DOI URL |
| [8] |
HE M C, YANG J R, 1999. Effects of different forms of antimony on rice during the period of germination and growth and antimony concentration in rice tissue[J]. Science of the Total Environment, 243-244: 149-155.
DOI URL |
| [9] |
JORMAKKA M, TöRNROTH S, BYRNE B, et al., 2002. Molecular basis of proton motive force generation: Structure of formate dehydrogenase-N[J]. Science, 295(5561): 1863-1868.
PMID |
| [10] | KIELKOPF C L, BAUER W, URBATSCH I L, 2020. Bradford assay for determining protein concentration[M]. Cold Spring Harbor Protocols, (4): 102269. |
| [11] |
KLÜPFEL L, PIEPENBROCK A, KAPPLER A, et al., 2014. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments[J]. Nature Geoscience, 7(3): 195-200.
DOI |
| [12] |
LI Q, HUANG M H, SHU S H, et al., 2022. Quinone-mediated Sb removal from sulfate-rich wastewater by anaerobic granular sludge: Performance and mechanisms[J]. Science of The Total Environment, 838(Part 3): 156217.
DOI URL |
| [13] |
MENG Y L, LIU Z J, ROSEN B P, 2004. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli[J]. Journal of Biological Chemistry, 279(18): 18334-18341.
DOI URL |
| [14] | MORENO-VIVIáN C, FLORES E, 2007. Chapter 17 - nitrate assimilation in bacteria[M]// Biology of the Nitrogen Cycle. Amsterdam: Elsevier: 263-282. |
| [15] |
MURCIEGO A M, SÁNCHEZ A G, GONZÁLEZ M A R, et al., 2007. Antimony distribution and mobility in topsoils and plants (Cytisus striatus, Cistus ladanifer and Dittrichia viscosa) from polluted Sb-mining areas in Extremadura (Spain)[J]. Environmental Pollution, 145(1): 15-21.
DOI PMID |
| [16] |
NGUYEN V K, PARK Y, LEE T, 2019. Microbial antimonate reduction with a solid-state electrode as the sole electron donor: A novel approach for antimony bioremediation[J]. Journal of Hazardous Materials, 377: 179-185.
DOI PMID |
| [17] | OKAMOTO A, SAITO K, INOUE K, et al., 2014. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species[J]. Energy & Environmental Science, 7(4): 1357-1361. |
| [18] |
PAT-ESPADAS A M, RAZO-FLORES E, RANGEL-MENDEZ J R, et al., 2014. Direct and quinone-mediated palladium reduction by geobacter sulfurreducens: Mechanisms and modeling[J]. Environmental Science & Technology, 48(5): 2910-2919.
DOI URL |
| [19] |
SAINI G, CHAN C S, 2012. Near-neutral surface charge and hydrophilicity prevent mineral encrustation of Fe-oxidizing micro-organisms[J]. Geobiology, 11(2): 191-200.
DOI URL |
| [20] |
SCHEINOST A C, ROSSBERG A, VANTELON D, et al., 2006. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy[J]. Geochimica et Cosmochimica Acta, 70(13): 3299-3312.
DOI URL |
| [21] |
SHI L D, WANG M, HAN Y L, et al., 2019. Multi-omics reveal various potential antimonate reductases from phylogenetically diverse microorganisms[J]. Applied Microbiology and Biotechnology, 103(21): 9119-9129.
DOI |
| [22] |
SUN W M, SUN X X, HAGGBLOM M M, et al., 2021. Identification of antimonate reducing bacteria and their potential metabolic traits by the combination of stable isotope probing and metagenomic-pangenomic analysis[J]. Environmental Science and Technology, 55(20): 13902-13912.
DOI URL |
| [23] | THÖNY-MEYER L, 1997. Biogenesis of respiratory cytochromes in bacteria[J]. Microbiology and Molecular Biology Reviews, 61(3): 337-376. |
| [24] | TOLAR JOE G, LI S, AJO-FRANKLIN CAROLINE M, 2022. The differing roles of flavins and quinones in extracellular electron transfer in lactiplantibacillus plantarum[J]. Applied and Environmental Microbiology, 89(1): e01313-01322. |
| [25] |
WANG L Y, YE L, JING C Y, 2020. Genetic identification of antimonate respiratory reductase in Shewanella sp. ANA-3[J]. Environmental Science & Technology, 54(21): 14107-14113.
DOI URL |
| [26] |
WANG L Y, YE L, YU Y Q, et al., 2018. Antimony redox biotransformation in the subsurface: Effect of indigenous Sb(V) respiring microbiota[J]. Environmental Science & Technology, 52(3): 1200-1207.
DOI URL |
| [27] |
WANG S S, XING Z H, CHEN G Y, et al., 2016. Cuboctahedral Sb2O3 mesocrystals organized from octahedral building blocks: More than self-similarity[J]. Crystal Growth & Design, 16(7): 3613-3617.
DOI URL |
| [28] |
WANG X M, WANG L, CHEN L, et al., 2022. AQDS activates extracellular synergistic biodetoxification of copper and selenite via altering the coordination environment of outer-membrane proteins[J]. Environmental Science & Technology, 56(19): 13786-13797.
DOI URL |
| [29] |
WANG X Q, LI F B, YUAN C L, et al., 2019. The translocation of antimony in soil-rice system with comparisons to arsenic: Alleviation of their accumulation in rice by simultaneous use of Fe(II) and NO3-[J]. Science of the Total Environment, 650(Part 1): 633-641.
DOI URL |
| [30] |
WU Y, LUO X, QIN B, et al., 2020. Enhanced current production by exogenous electron mediators via synergy of promoting biofilm formation and the electron shuttling process[J]. Environmental Science & Technology, 54(12): 7217-7225.
DOI URL |
| [31] |
YAMAMURA S, IIDA C, KOBAYASHI Y, et al., 2021. Production of two morphologically different antimony trioxides by a novel antimonate-reducing bacterium, Geobacter sp. SVR[J]. Journal of Hazardous Materials, 411: 125100.
DOI URL |
| [32] |
YANG Z R, HOSOKAWA H, SADAKANE T, et al., 2020. Isolation and characterization of facultative-anaerobic antimonate-reducing bacteria[J]. Microorganisms, 8(9): 1435.
DOI URL |
| [33] | YING Z Y, CHEN H, HE Z, et al., 2022. Redox mediator-regulated microbial electrolysis cell to boost coulombic efficiency and degradation activity during gaseous chlorobenzene abatement[J]. Journal of Power Sources, 528: 231314. |
| [34] |
YU H, YAN X Z, WENG W L, et al., 2022. Extracellular proteins of Desulfovibrio vulgaris as adsorbents and redox shuttles promote biomineralization of antimony[J]. J Hazard Mater, 426: 127795.
DOI URL |
| [35] |
YU Y S, CHEN J C, LI Y P, et al., 2021. Identification of a MarR subfamily that regulates arsenic resistance genes[J]. Applied and Environmental Microbiology, 87(24): e0158821.
DOI URL |
| [36] |
ZHANG Y D, BOYANOV M I, O’LOUGHLIN E J, et al., 2024. Reaction pathways and Sb(III) minerals formation during the reduction of Sb(V) by Rhodoferax ferrireducens strain YZ-1[J]. Journal of Hazardous Materials, 465: 133240.
DOI URL |
| [37] |
ZHOU J Z, WU C Y, PANG S, et al., 2022a. Dissimilatory and cytoplasmic antimonate reductions in a hydrogen-based membrane biofilm reactor[J]. Environmental Science & Technology, 56(20): 14808-14816.
DOI URL |
| [38] |
ZHOU J Z, WU C Y, PANG S, et al., 2022b. Dissimilatory and cytoplasmic antimonate reductions in a hydrogen-based membrane biofilm reactor[J]. Environmental Science & Technology, 56(20): 14808-14816.
DOI URL |
| [39] |
ZOTOV A V, SHIKINA N D, AKINFIEV N N, 2003. Thermodynamic properties of the Sb(III) hydroxide complex Sb(OH)3(aq) at hydrothermal conditions[J]. Geochimica et Cosmochimica Acta, 67(10): 1821-1836.
DOI URL |
| [40] |
阳涅, 孙晓旭, 孔天乐, 等, 2023. 微生物群落对河流底泥中锑含量变化的响应[J]. 生态环境学报, 32(3): 609-618.
DOI |
| YANG N, SUN X X, KONG T L, et al.., 2023. Response of microbial communities to changes in antimony content in river sediments[J]. Journal of Ecology and Environment, 32(3): 609-618. |
| [1] | 丁昊, 李长鑫, 丁静, 兰昊. n-damo细菌在不同生态环境中的遗传多样性和潜在功能研究[J]. 生态环境学报, 2024, 33(2): 202-211. |
| [2] | 李嘉惠, 童辉, 陈曼佳, 刘承帅, 姜琪, 易秀. 微氧生物亚铁氧化及其重金属固定效应研究进展[J]. 生态环境学报, 2024, 33(2): 310-320. |
| [3] | 马媛, 田路露, 吕杰, 柳沛, 张旭, 李二阳, 张清航. 天山北坡雪岭云杉森林土壤微生物群落及影响因素研究[J]. 生态环境学报, 2024, 33(1): 1-11. |
| [4] | 杨正桥, 邹奇, 韦行, 周凯, 陈志良. 金属尾矿微生物对尾矿环境的适应与调控机制研究进展[J]. 生态环境学报, 2024, 33(1): 156-166. |
| [5] | 袁佳宝, 宋艳宇, 刘桢迪, 朱梦圆, 程小峰, 马秀艳, 陈宁, 李晓宇. 松嫩平原芦苇湿地土壤酶活性剖面分布特征及其微生物养分限制指示作用[J]. 生态环境学报, 2023, 32(12): 2141-2153. |
| [6] | 李成涛, 吴婉晴, 陈晨, 张勇, 张凯. 可生物降解PBAT微塑料对土壤理化性质及上海青生理指标的影响[J]. 生态环境学报, 2023, 32(11): 1964-1977. |
| [7] | 李璇, 钱秀雯, 黄娟, 王鸣宇, 肖君. 纳米氧化镍暴露下人工湿地运行性能及微生物群落的响应[J]. 生态环境学报, 2023, 32(10): 1833-1841. |
| [8] | 梁川, 杨艳芳, 俞姗姗, 周利, 张经纬, 张秀娟. 围网与围塘养鱼下沉积物微生物量和群落结构特征差异[J]. 生态环境学报, 2023, 32(10): 1802-1810. |
| [9] | 唐志伟, 翁颖, 朱夏童, 蔡洪梅, 代雯慈, 王捧娜, 郑宝强, 李金才, 陈翔. 秸秆还田下中国农田土壤微生物生物量碳变化及其影响因素的Meta分析[J]. 生态环境学报, 2023, 32(9): 1552-1562. |
| [10] | 梁川, 杨艳芳, 俞姗姗, 周利, 张经纬, 张秀娟. 围网与围塘养鱼下沉积物微生物量和群落结构特征差异[J]. 生态环境学报, 2023, 32(8): 1487-1495. |
| [11] | 姜懿珊, 孙迎韬, 张干, 罗春玲. 中国不同气候类型森林土壤微生物群落结构及其影响因素[J]. 生态环境学报, 2023, 32(8): 1355-1364. |
| [12] | 朱忆雯, 尹丹, 胡敏, 杜衍红, 洪泽彬, 程宽, 于焕云. 稻田土壤氮循环与砷形态转化耦合的研究进展[J]. 生态环境学报, 2023, 32(7): 1344-1354. |
| [13] | 陈懂懂, 霍莉莉, 赵亮, 陈昕, 舒敏, 贺福全, 张煜坤, 张莉, 李奇. 青海高寒草地水热因子对土壤微生物生物量碳、氮空间变异的贡献——基于增强回归树模型[J]. 生态环境学报, 2023, 32(7): 1207-1217. |
| [14] | 李桂英, 刘建莹, 安太成. 水体消毒过程中活的不可培养细菌的形成与复苏机制研究进展[J]. 生态环境学报, 2023, 32(7): 1333-1343. |
| [15] | 寇祝, 卿纯, 袁昌果, 李平. 西藏东北部热泉水中硫氧化菌的多样性及分布特征[J]. 生态环境学报, 2023, 32(5): 989-1000. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
