生态环境学报 ›› 2024, Vol. 33 ›› Issue (4): 585-596.DOI: 10.16258/j.cnki.1674-5906.2024.04.009
收稿日期:2024-02-01
出版日期:2024-04-18
发布日期:2024-05-31
通讯作者:
*袁勇。E-mail: yyuan2017@gdut.edu.cn作者简介:张仪春(1999年生),女,硕士研究生,研究方向为水热腐殖化人工腐殖质。E-mail: 1240620966@qq.com
基金资助:
ZHANG Yichun1,2( ), YU Zhen3, YUAN Yong1,2,*(
), YU Zhen3, YUAN Yong1,2,*( )
)
Received:2024-02-01
Online:2024-04-18
Published:2024-05-31
摘要:
腐殖质(HS)是由动植物尸体分解和微生物缓慢转化而成的天然有机物质,其快速制备可通过生物质的水热处理实现,但人工腐殖质的光谱和电化学特性研究仍然不足。运用水热腐殖化法以玉米秸秆为原料制备人工胡敏酸(AHA)和富里酸(AFA)。通过光谱学和高分辨质谱分析了其化学结构和分子组成,并与土壤和泥炭中的胡敏酸和富里酸做了对比。利用电化学分析测定了HS的电子接受能力(EAC)和电子供给能力(EDC)。光谱和质谱结果揭示人工HS与天然HS在化学结构上高度一致,具有相同的主要化学基团,包括羟基、脂肪族、芳香族和羧酮基团。此外,人工和天然HS均较高的含有木质素/富羧酸的脂环结构、脂类和脂肪族/蛋白质,但人工HS具有较高的荧光指数和较低的芳香程度,富含类酪氨酸荧光组分。电化学结果表明,AHA的EAC为1.85 mmol·g-1,高于天然胡敏酸;其EDC为0.5 mmol·g-1,与天然胡敏酸相当。AFA的EAC为2.07 mmol·g-1,低于天然富里酸,但EDC为0.66 mmol·g-1,高于天然富里酸。相关性分析表明,木质素/富羧基脂环结构(CRAM)和脂质含量对电子转移能力有显著影响。类酪氨酸荧光成分、含氮有机化合物(CHON)与EDC分别呈正相关关系,而类腐殖质荧光组分、原子O/C值及双键当量(DBE)与EAC呈正相关关系。综上,人工腐殖质不仅表现出良好的电化学性能,提升了对人工腐殖质在模拟天然HS功能及其在地球化学电子转移过程中潜在应用的理解。
中图分类号:
张仪春, 余震, 袁勇. 水热腐殖化人工腐殖质的光谱和电化学特性研究[J]. 生态环境学报, 2024, 33(4): 585-596.
ZHANG Yichun, YU Zhen, YUAN Yong. Spectroscopic and Electrochemical Characteristics of Artificial Humic Substances Produced by Hydrothermal Humification[J]. Ecology and Environment, 2024, 33(4): 585-596.
| 腐殖质样品 | AHA | SHA | PHA | AFA | SFA | PFA |
|---|---|---|---|---|---|---|
| SUVA254 | 0.057 | 0.068 | 0.056 | 0.011 | 0.048 | 0.047 |
| w(C)/% | 62.81 | 46.41 | 49.85 | 47.23 | 42.36 | 46.69 |
| w(H)/% | 6.14 | 4.14 | 4.34 | 5.72 | 4.54 | 4.52 |
| w(O)/% | 26.91 | 44.14 | 41.95 | 45.12 | 47.78 | 45.1 |
| w(N)/% | 2.54 | 4.36 | 2.72 | 1.87 | 4.72 | 2.46 |
| w(S)/% | 0.38 | 0.44 | 0.49 | 0.57 | 0.47 | 0.37 |
| H/C | 0.098 | 0.089 | 0.087 | 0.121 | 0.107 | 0.097 |
| O/C | 0.43 | 0.95 | 0.84 | 0.96 | 1.13 | 0.97 |
| N/C | 0.040 | 0.094 | 0.055 | 0.040 | 0.111 | 0.053 |
| HIX | 0.67 | 0.28 | 0.14 | 0.56 | 0.2 | 0.18 |
| FI | 1.21 | 0.78 | 0.73 | 1.92 | 0.93 | 0.85 |
表1 腐殖质的光谱特征参数、元素组成及其比值
Table 1 Optical properties, elemental composition of humic substances
| 腐殖质样品 | AHA | SHA | PHA | AFA | SFA | PFA |
|---|---|---|---|---|---|---|
| SUVA254 | 0.057 | 0.068 | 0.056 | 0.011 | 0.048 | 0.047 |
| w(C)/% | 62.81 | 46.41 | 49.85 | 47.23 | 42.36 | 46.69 |
| w(H)/% | 6.14 | 4.14 | 4.34 | 5.72 | 4.54 | 4.52 |
| w(O)/% | 26.91 | 44.14 | 41.95 | 45.12 | 47.78 | 45.1 |
| w(N)/% | 2.54 | 4.36 | 2.72 | 1.87 | 4.72 | 2.46 |
| w(S)/% | 0.38 | 0.44 | 0.49 | 0.57 | 0.47 | 0.37 |
| H/C | 0.098 | 0.089 | 0.087 | 0.121 | 0.107 | 0.097 |
| O/C | 0.43 | 0.95 | 0.84 | 0.96 | 1.13 | 0.97 |
| N/C | 0.040 | 0.094 | 0.055 | 0.040 | 0.111 | 0.053 |
| HIX | 0.67 | 0.28 | 0.14 | 0.56 | 0.2 | 0.18 |
| FI | 1.21 | 0.78 | 0.73 | 1.92 | 0.93 | 0.85 |
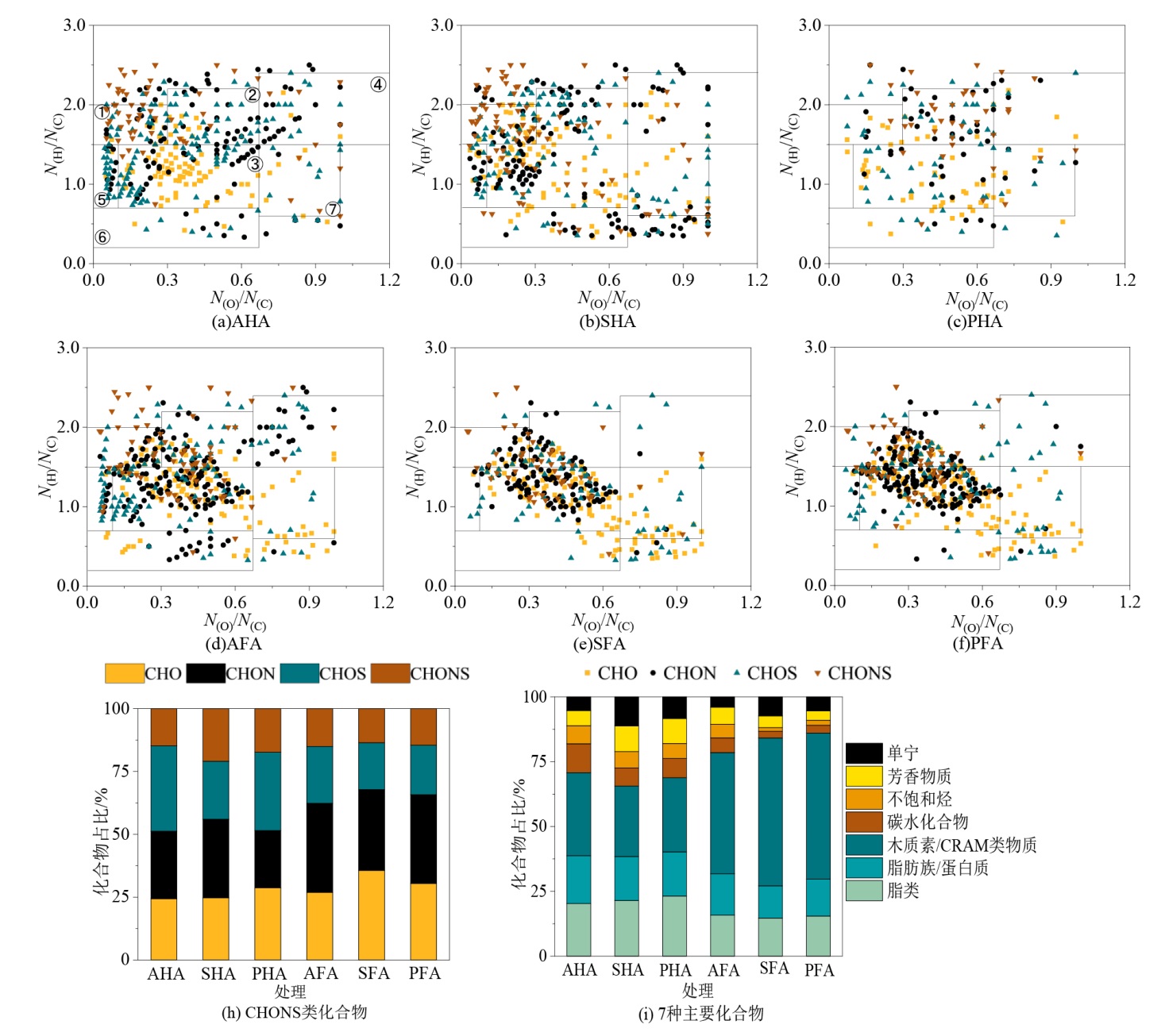
图3 腐殖质主要化合物类别的散点图及其相对丰度贡献图 N(H)/N(C)代表每个分子式的H与C原子数量的比值,N(O)/N(C)则代表每个分子式的O与C原子数量的比值;图a中的编号分别代表着1)脂类、2)脂肪族/蛋白质、3)木质素/富羧基脂环分子(CRAM)、4)碳水化物、5)不饱和烃、6)芳香结构和7)单宁
Figure 3 Molecular composition and its contribution of humic substances
| 腐殖质样品 | AHA | SHA | PHA | AFA | SFA | PFA |
|---|---|---|---|---|---|---|
| Formula number | 978 | 1076 | 556 | 1027 | 713 | 1065 |
| N(H)/N(C) | 1.13 | 0.98 | 1.44 | 1.02 | 0.98 | 1.03 |
| N(O)/N(C) | 0.45 | 0.58 | 0.33 | 0.57 | 0.59 | 0.57 |
| N(N)/N(C) | 0.036 | 0.044 | 0.027 | 0.016 | 0.013 | 0.014 |
| N(S)/N(C) | 0.012 | 0.021 | 0.007 | 0.007 | 0.008 | 0.008 |
| AImod | 0.35 | 0.40 | 0.18 | 0.33 | 0.35 | 0.32 |
| DBE | 17.84 | 19.91 | 13.05 | 22.19 | 23.15 | 22.25 |
| DBE/C | 0.49 | 0.57 | 0.33 | 0.53 | 0.55 | 0.52 |
表2 腐殖质样品分子组成的强度加权平均值
Table 2 Iintensity weighted averaged values for molecular composition of humic substances
| 腐殖质样品 | AHA | SHA | PHA | AFA | SFA | PFA |
|---|---|---|---|---|---|---|
| Formula number | 978 | 1076 | 556 | 1027 | 713 | 1065 |
| N(H)/N(C) | 1.13 | 0.98 | 1.44 | 1.02 | 0.98 | 1.03 |
| N(O)/N(C) | 0.45 | 0.58 | 0.33 | 0.57 | 0.59 | 0.57 |
| N(N)/N(C) | 0.036 | 0.044 | 0.027 | 0.016 | 0.013 | 0.014 |
| N(S)/N(C) | 0.012 | 0.021 | 0.007 | 0.007 | 0.008 | 0.008 |
| AImod | 0.35 | 0.40 | 0.18 | 0.33 | 0.35 | 0.32 |
| DBE | 17.84 | 19.91 | 13.05 | 22.19 | 23.15 | 22.25 |
| DBE/C | 0.49 | 0.57 | 0.33 | 0.53 | 0.55 | 0.52 |
| 名称 | 描述 | EAC/(mmol·g -1) | EDC/(mmol·g -1) | ETC/(mmol·g -1) | 来源 |
|---|---|---|---|---|---|
| AHA | 人工胡敏酸 | 1.85 | 0.50 | 2.35 | 实验数据 |
| SHA | 黑土胡敏酸 | 1.10 | 0.51 | 1.61 | 实验数据 |
| PHA | 泥炭土胡敏酸 | 0.71 | 0.49 | 1.20 | 实验数据 |
| AFA | 人工富里酸 | 2.07 | 0.66 | 2.73 | 实验数据 |
| SFA | 黑土富里酸 | 2.87 | 0.51 | 3.38 | 实验数据 |
| PFA | 泥炭土富里酸 | 2.26 | 0.53 | 2.79 | 实验数据 |
| AErShan-HA | 内蒙古阿尔山土壤胡敏酸 | 0.24 | 0.45 | 0.69 | Tan et al., |
| HA | 汕头砷污染稻田土 | 0.48 | 0.59 | 1.07 | Qiao et al., |
| CM-HA | 鸡粪堆肥提取的胡敏酸 | 2.50 | 0.54 | 3.04 | Zhao et al., |
| DCM-HA | 牛粪堆肥提取的胡敏酸 | 1.56 | 0.60 | 2.16 | Zhao et al., |
| FVW-HA | 果蔬堆肥提取的胡敏酸 | 0.93 | 0.91 | 1.83 | Zhao et al., |
| WW-HA | 杂草堆肥提取的胡敏酸 | 0.92 | 0.85 | 1.76 | Zhao et al., |
| SW-HA | 玉米秸秆堆肥提取的胡敏酸 | 1.77 | 0.82 | 2.59 | Zhao et al., |
| SS-HA | 污水污泥堆肥提取的胡敏酸 | 1.77 | 0.66 | 2.43 | Zhao et al., |
| AErShan-FA | 内蒙古阿尔山土壤富里酸 | 0.26 | 0.27 | 0.53 | Tan et al., |
| FA | 汕头砷污染稻田土 | 0.62 | 0.54 | 1.16 | Qiao et al., |
| CM-FA | 鸡粪堆肥提取的富里酸 | 1.18 | 0.80 | 1.98 | Zhao et al., |
| DCM-FA | 牛粪堆肥提取的富里酸 | 1.08 | 0.88 | 1.96 | Zhao et al., |
| FVW-FA | 果蔬堆肥提取的富里酸 | 1.36 | 1.31 | 2.68 | Zhao et al., |
| WW-FA | 杂草堆肥提取的富里酸 | 1.44 | 0.64 | 2.08 | Zhao et al., |
| SW-FA | 玉米秸秆堆肥提取的富里酸 | 1.33 | 0.50 | 1.83 | Zhao et al., |
| SS-FA | 污水污泥堆肥提取的富里酸 | 1.06 | 0.99 | 2.05 | Zhao et al., |
| HA | 胡敏酸电子转移能力均值 | 1.26 | 0.63 | 1.88 | |
| FA | 富里酸电子转移能力均值 | 1.41 | 0.69 | 2.11 |
表3 不同腐殖质的接受电子和供给电子能力
Table 3 EAC and EDC of various humic substances
| 名称 | 描述 | EAC/(mmol·g -1) | EDC/(mmol·g -1) | ETC/(mmol·g -1) | 来源 |
|---|---|---|---|---|---|
| AHA | 人工胡敏酸 | 1.85 | 0.50 | 2.35 | 实验数据 |
| SHA | 黑土胡敏酸 | 1.10 | 0.51 | 1.61 | 实验数据 |
| PHA | 泥炭土胡敏酸 | 0.71 | 0.49 | 1.20 | 实验数据 |
| AFA | 人工富里酸 | 2.07 | 0.66 | 2.73 | 实验数据 |
| SFA | 黑土富里酸 | 2.87 | 0.51 | 3.38 | 实验数据 |
| PFA | 泥炭土富里酸 | 2.26 | 0.53 | 2.79 | 实验数据 |
| AErShan-HA | 内蒙古阿尔山土壤胡敏酸 | 0.24 | 0.45 | 0.69 | Tan et al., |
| HA | 汕头砷污染稻田土 | 0.48 | 0.59 | 1.07 | Qiao et al., |
| CM-HA | 鸡粪堆肥提取的胡敏酸 | 2.50 | 0.54 | 3.04 | Zhao et al., |
| DCM-HA | 牛粪堆肥提取的胡敏酸 | 1.56 | 0.60 | 2.16 | Zhao et al., |
| FVW-HA | 果蔬堆肥提取的胡敏酸 | 0.93 | 0.91 | 1.83 | Zhao et al., |
| WW-HA | 杂草堆肥提取的胡敏酸 | 0.92 | 0.85 | 1.76 | Zhao et al., |
| SW-HA | 玉米秸秆堆肥提取的胡敏酸 | 1.77 | 0.82 | 2.59 | Zhao et al., |
| SS-HA | 污水污泥堆肥提取的胡敏酸 | 1.77 | 0.66 | 2.43 | Zhao et al., |
| AErShan-FA | 内蒙古阿尔山土壤富里酸 | 0.26 | 0.27 | 0.53 | Tan et al., |
| FA | 汕头砷污染稻田土 | 0.62 | 0.54 | 1.16 | Qiao et al., |
| CM-FA | 鸡粪堆肥提取的富里酸 | 1.18 | 0.80 | 1.98 | Zhao et al., |
| DCM-FA | 牛粪堆肥提取的富里酸 | 1.08 | 0.88 | 1.96 | Zhao et al., |
| FVW-FA | 果蔬堆肥提取的富里酸 | 1.36 | 1.31 | 2.68 | Zhao et al., |
| WW-FA | 杂草堆肥提取的富里酸 | 1.44 | 0.64 | 2.08 | Zhao et al., |
| SW-FA | 玉米秸秆堆肥提取的富里酸 | 1.33 | 0.50 | 1.83 | Zhao et al., |
| SS-FA | 污水污泥堆肥提取的富里酸 | 1.06 | 0.99 | 2.05 | Zhao et al., |
| HA | 胡敏酸电子转移能力均值 | 1.26 | 0.63 | 1.88 | |
| FA | 富里酸电子转移能力均值 | 1.41 | 0.69 | 2.11 |
| [1] | AESCHBACHER M, GRAF C, SCHWARZENBACH R P, et al., 2012. Antioxidant properties of humic substances[J]. Environmental Science & Technology, 46(9): 4916-4925. |
| [2] | AESCHBACHER M, VERGARI D, SCHWARZENBACH R P, et al., 2011. Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids[J]. Environmental Science & Technology, 45(19): 8385-8394. |
| [3] | AI S, MENG X, ZHANG Z, et al., 2023. Artificial humic acid regulates the impact of fungal community on soil macroaggregates formation[J]. Chemosphere, 332: 138822. |
| [4] | CHEN M L, LIU S S, BI M H, et al., 2022a. Aging behavior of microplastics affected DOM in riparian sediments: From the characteristics to bioavailability[J]. Journal of Hazardous Materials, 431: 128522. |
| [5] |
CHEN P C, TAO S T, ZHENG P, 2016. Efficient and repeated production of succinic acid by turning sugarcane bagasse into sugar and support[J]. Bioresource Technology, 211: 406-413.
DOI PMID |
| [6] | CHEN P F, YANG R J, PEI Y H, et al., 2022b. Hydrothermal synthesis of similar mineral-sourced humic acid from food waste and the role of protein[J]. Science of the Total Environment, 828: 154440. |
| [7] | CORY R M, MCKNIGHT D M, 2005. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter[J]. Environmental Science & Technology, 39(21): 8142-8149. |
| [8] | D’ANDRILLI J, SILVERMAN V, BUCKLEY S, et al., 2022. Inferring ecosystem function from dissolved organic matter optical properties: A critical review[J]. Environmental Science & Technology, 56(16): 11146-11161. |
| [9] | DARGIE G C, LEWIS S L, LAWSON I T, et al., 2017. Age, extent and carbon storage of the central Congo Basin peatland complex[J]. Nature, 542(7639): 86-90. |
| [10] | DEMIR-CAKAN R, BACCILE N, ANTONIETTI M, et al., 2009. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid[J]. Chemistry of Materials, 21(3): 484-490. |
| [11] | ESPOSITO D, ANTONIETTI M, 2013. Chemical conversion of sugars to lactic acid by alkaline hydrothermal processes[J]. Chemistry Sustainability Energy Materials, 6(6): 989-992. |
| [12] | FELLMAN J B, HOOD E, SPENCER R G M, 2010. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review[J]. Limnology and Oceanography, 55(6): 2452-2462. |
| [13] | GUO X X, LIU H T, WU S B, 2019. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions[J]. Science of the total Environment, 662: 501-510. |
| [14] | GUTH P, GAO C Y, KNORR K H, 2023. Electron accepting capacities of a wide variety of peat materials from around the globe similarly explain CO2 and CH4 formation[J]. Global Biogeochemical Cycles, 37(1): 20. |
| [15] | HAN R X, WANG Z, LÜ J T, et al., 2022. Multiple effects of humic components on microbially mediated iron redox processes and production of hydroxyl radicals[J]. Environmental Science & Technology, 56(22): 16419-16427. |
| [16] | HE W J, ZHONG Q F, LIU J Y, et al., 2023. Microbially mediated molecular transformations of dissolved organic matter in bioelectrochemical systems treating beer brewery wastewater[J]. Chemical Engineering Journal, 461: 142111. |
| [17] | HE X S, XI B D, CUI D Y, et al., 2014. Influence of chemical and structural evolution of dissolved organic matter on electron transfer capacity during composting[J]. Journal of Hazardous Materials, 268: 256-263. |
| [18] | HU B, WANG K, WU L H, et al., 2010. Engineering carbon materials from the hydrothermal carbonization process of biomass[J]. Advanced Materials, 22(7): 813-828. |
| [19] | ISHII S K L, BOYER T H, 2012. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: a critical review[J]. Environmental Science & Technology, 46(4): 2006-2017. |
| [20] | JIANG J, KAPPLER A, 2008. Kinetics of microbial and chemical reduction of humic substances: Implications for electron shuttling[J]. Environmental Science & Technology, 42(10): 3563-3569. |
| [21] | JIANG T, WEI S Q, FLANAGAN D C, et al., 2014. Effect of abiotic factors on the mercury reduction process by humic acids in aqueous systems[J]. Pedosphere, 24(1): 125-136. |
| [22] | KANG S H, CHOI W, 2009. Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle[J]. Environmental Science & Technology, 43(3): 878-883. |
| [23] | KLÜPFEL L, PIEPENBROCK A, KAPPLER A, et al., 2014. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments[J]. Nature Geoscience, 7(3): 195-200. |
| [24] | LI L, PHUNGSAI P, KURISU F, et al., 2021. Orbitrap mass spectrometry for the molecular characterization of water resource recovery from polluted surface water using membrane bioreactor[J]. Chemosphere, 270: 128771. |
| [25] | LI W, LI X, HAN C X, et al., 2023. A new view into three-dimensional excitation-emission matrix fluorescence spectroscopy for dissolved organic matter[J]. Science of the Total Environment, 855: 158963. |
| [26] | LOVLEY D R, COATES J D, BLUNT-HARRIS E L, et al., 1996. Humic substances as electron acceptors for microbial respiration[J]. Nature, 382(6590): 445-448. |
| [27] | LU Y, HU S W, ZHANG H Y, et al., 2022. Effect of humic acid on bioreduction of facet-dependent hematite by Shewanella putrefaciens CN-32[J]. Science of the Total Environment, 849: 157713. |
| [28] |
MANGAL V, STOCK N L, GUÉGUEN C, 2016. Molecular characterization of phytoplankton dissolved organic matter (DOM) and sulfur components using high resolution Orbitrap mass spectrometry[J]. Analytical and Bioanalytical Chemistry, 408(7): 1891-1900.
DOI PMID |
| [29] |
MYNENI S C B, BROWN J T, MARTINEZ G A, et al., 1999. Imaging of humic substance macromolecular structures in water and soils[J]. Science, 286(5443): 1335-1337.
PMID |
| [30] | NIE J X, YAN S W, LIAN L S, et al., 2020. Development of fluorescence surrogates to predict the ferrate (VI) oxidation of pharmaceuticals in wastewater effluents[J]. Water Research, 185: 116256. |
| [31] | OU J J, WEN J L, TAN W B, et al., 2023. A data-driven approach for understanding the structure dependence of redox activity in humic substances[J]. Environmental Research, 219: 115142. |
| [32] |
PANDEY A K, PANDEY S D, MISRA V, 2000. Stability constants of metal-humic acid complexes and its role in environmental detoxification[J]. Ecotoxicology and Environmental Safety, 47(2): 195-200.
PMID |
| [33] | PICCOLO A, PIETRAMELLARA G, MBAGWU J S C, 1997. Use of humic substances as soil conditioners to increase aggregate stability[J]. Geoderma, 75(3-4): 267-277. |
| [34] | QIAO J, LI X M, LI F B, et al., 2019. Humic substances facilitate arsenic reduction and release in flooded paddy soil[J]. Environmental Science & Technology, 53(9): 5034-5042. |
| [35] | SHAO Y V, BAO M G, HUO W Z, et al., 2022. Production of artificial humic acid from biomass residues by a non-catalytic hydrothermal process[J]. Journal of Cleaner Production, 335: 130302. |
| [36] | STERN N, MEJIA J, HE S, et al., 2018. Dual role of humic substances as electron donor and shuttle for dissimilatory iron reduction[J]. Environmental Science & Technology, 52(10): 5691-5699. |
| [37] | SUN B, LI Y S, SONG M J, et al., 2022. Molecular characterization of the composition and transformation of dissolved organic matter during the semi-permeable membrane covered hyperthermophilic composting[J]. Journal of Hazardous Materials, 425: 127496. |
| [38] | TAN W B, XI B D, WANG G A, et al., 2017. Increased electron-accepting and decreased electron-donating capacities of soil humic substances in response to increasing temperature[J]. Environmental Science & Technology, 51(6): 3176-3186. |
| [39] | TANG C Y, CHENG K, LIU B L, et al., 2022. Artificial humic acid facilitates biological carbon sequestration under freezing-thawing conditions[J]. Science of the Total Environment, 849: 157841. |
| [40] | TANG C Y, LI Y L, SONG J P, et al., 2021. Artificial humic substances improve microbial activity for binding CO2[J]. Iscience, 24(6): 102647. |
| [41] | WANG C Q, CHENG T F, ZHANG D Y, et al., 2023. Electrochemical properties of humic acid and its novel applications: A tip of the iceberg[J]. Science of the Total Environment, 863: 160755. |
| [42] | WANG W, HE C, GAO Y, et al., 2019. Isolation and characterization of hydrophilic dissolved organic matter in waters by ion exchange solid phase extraction followed by high resolution mass spectrometry[J]. Environmental Chemistry Letters, 17: 1857-1866. |
| [43] | WEISHAAR J L, AIKEN G R, BERGAMASCHI B A, et al., 2003. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon[J]. Environmental Science & Technology, 37(20): 4702-4708. |
| [44] | WU F C, EVANS R D, DILLON P J, 2003. Separation and characterization of NOM by high-performance liquid chromatography and on-line three-dimensional excitation emission matrix fluorescence detection[J]. Environmental Science & Technology, 37(16): 3687-3693. |
| [45] | YANG C, HOU L X, XI B D, et al., 2022. Contribution of redox-active properties of compost-derived humic substances in hematite bioreduction[J]. Chinese Chemical Letters, 33(5): 2731-2735. |
| [46] | YANG F, ANTONIETTI M, 2020a. Artificial humic acids: Sustainable materials against climate change[J]. Advanced Science, 7(5): 1902992. |
| [47] | YANG F, ANTONIETTI M, 2020b. The sleeping giant: A polymer View on humic matter in synthesis and applications[J]. Progress in Polymer Science, 100: 101182. |
| [48] | YANG F, ZHANG S S, CHENG K, et al., 2019a. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation[J]. Science of the Total Environment, 686: 1140-1151. |
| [49] | YANG F, ZHANG S S, SONG J P, et al., 2019b. Synthetic humic acids solubilize otherwise insoluble phosphates to improve soil fertility[J]. Angewandte Chemie, 131(52): 18989-18992. |
| [50] |
YANG X F, WANG H M, LI C, et al., 2017. Restoring of glucose metabolism of engineered Yarrowia lipolytica for succinic acid production via a simple and efficient adaptive evolution strategy[J]. Journal of Agricultural and Food Chemistry, 65(20): 4133-4139.
DOI PMID |
| [51] | YUAN Z, HE C, SHI Q, et al., 2017. Molecular insights into the transformation of dissolved organic matter in landfill leachate concentrate during biodegradation and coagulation processes using ESI FT-ICR MS[J]. Environmental Science & Technology, 51(14): 8110-8118. |
| [52] | ZHANG B P, ZHOU S F, ZHOU L H, et al., 2019. Pyrolysis temperature-dependent electron transfer capacities of dissolved organic matters derived from wheat straw biochar[J]. Science of the Total Environment, 696: 133895. |
| [53] | ZHANG S S, SONG J P, DU Q, et al., 2020. Analog synthesis of artificial humic substances for efficient removal of mercury[J]. Chemosphere, 250: 126606. |
| [54] |
ZHAO X Y, HE X S, XI B D, et al., 2017. Response of humic-reducing microorganisms to the redox properties of humic substance during composting[J]. Waste Management, 70: 37-44.
DOI PMID |
| [55] | ZHI Y C, LI X N, LIAN F, et al., 2022. Nanoscale Iron trioxide catalyzes the synthesis of auxins analogs in artificial humic acids to enhance rice growth[J]. Science of the Total Environment, 848: 157536. |
| [56] | ZHOU S F, LIAO Z Y, ZHANG B P, et al., 2021. Photochemical behavior of microbial extracellular polymeric substances in the aquatic environment[J]. Environmental Science & Technology, 55(22): 15090-15099. |
| [57] | ZHU J, LI M, WHELAN M, 2018. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review[J]. Science of the Total Environment, 612: 522-537. |
| [58] | 崔东宇, 何小松, 席北斗, 等, 2015. 堆肥腐熟前后胡敏酸与富里酸的还原容量比较[J]. 中国环境科学, 35(7): 2087-2094. |
| CUI D Y, HE X S, XI B D, et al., 2015. The comparison of reduction capacity between humic acid and fulvic acid extracted from the compost[J]. China Environmental Science, 35(7): 2087-2094. | |
| [59] | 黄莎, 王彬, 曾丹, 等, 2020. 不同源胡敏酸的表征及其对磺胺嘧啶光降解的影响[J]. 环境科学学报, 40(1): 260-268. |
| HUANG S, WANG B, ZENG D, et al., 2020. Characterization of humic acid from different sources and its effection photodegradation of sulfadiazine[J]. Acta Scientiae Circumstantiae, 40(1): 260-268. | |
| [60] | 李柯蒙, 李洁月, 游少鸿, 等, 2022. 猪粪堆肥过程中腐殖酸电子转移机制及光谱演化特征[J]. 环境工程, 40(12): 79-88. |
| LI K M, LI J Y, YOU S H, et al., 2022. Electron transfer mechanism and spectral evolution characteristics of humic acids during pig manure composting[J]. Environmental Engineering, 40(12): 79-88. | |
| [61] | 柳广飞, 朱佳琪, 于华莉, 等, 2018. 电子穿梭体介导微生物还原铁氧化物的研究进展[J]. 地球科学, 43(S1): 157-170. |
| LIU F Y, ZHU J Q, YU H L, et al., 2018. Review on electron- shuttle-mediated microbial reduction of iron oxides minerals[J]. Earth Science, 43(S1): 157-170. | |
| [62] | 肖骁, 何小松, 席北斗, 等, 2018. 生活垃圾填埋富里酸电子转移能力与影响因素[J]. 环境化学, 37(4): 679-688. |
| XIAO X, HE X S, XI B D, et al., 2018. Electron transfer capacity of fulvic acid and its factors during municipal solid waste landfill[J]. Environmental Chemistry, 37(4): 679-688. | |
| [63] | 杨超, 何小松, 席北斗, 等, 2016. 垃圾填埋初期水溶性有机物电子转移能力特征研究[J]. 分析化学, 44(10): 1568-1574. |
| YANG C, HE X S, XI B D, et al., 2016. Characteristic study of dissolved organic matter for electron transfer capacity during initial landfill stage[J]. Chinese Journal of Analytical Chemistry, 44(10): 1568-1574. | |
| [64] |
张睿含, 智燕彩, 贾明昊, 等, 2023. 生物质废弃物类型和水热pH对人工腐殖酸性能影响[J]. 生态环境学报, 32(8): 1496-1506.
DOI |
| ZHANG R H, ZHI Y C, JIA M H, et al., 2023. Effects of feedstock types and hydrothermal solution pH on the properties of artificial humic acids[J]. Ecology and Environmental Sciences, 32(8): 1496-1506. | |
| [65] |
张玉龙, 陈雪丽, 吴云当, 2021. 电子穿梭体及其介导的环境与地球化学过程研究进展[J]. 生态环境学报, 30(1): 213-222.
DOI |
| ZHANG Y L, CHEN X L, WU Y D, 2021. Electron shuttle-mediated microbial extracellular electron transfer: Mechanisms and geochemical implications[J]. Ecology and Environmental Sciences, 30(1): 213-222. | |
| [66] | 赵秀云, 2021. 玉米秸秆堆肥木质素酚类型及微生物对腐殖酸电子转移能力影响研究[D]. 北京: 中国环境科学研究院. |
| ZHAO X Y, 2021. Study on effect of lignophenol type and microbes on electron transfer capacity of humic substances during corn stalks compost[D]. Beijing: Chinese Research Academy of Environmental Sciences. |
| [1] | 王室苹, 李梅, 安娅, 秦好丽. 镁改性增强小麦秸秆生物炭对镉的吸附能力:表面络合模型研究[J]. 生态环境学报, 2024, 33(4): 617-625. |
| [2] | 闫兴蕊, 龚平, 王小萍, 商立海, 李一农, 毛飞剑, 牛学锐, 张勃. 三江源地区土壤和牧草中的有机氯污染物:分布、来源和生态风险[J]. 生态环境学报, 2024, 33(3): 428-438. |
| [3] | 李高帆, 徐文卓, 卫昊明, 晏再生, 尤佳, 江和龙, 黄娟. 三维多孔生物炭吸附剂的制备及其对菲的吸附行为[J]. 生态环境学报, 2024, 33(2): 261-271. |
| [4] | 周舒, 于冰洋, 杜柯龙, 林榆文, 冯能佳, 智丹. 电化学氧化降解水中三唑酮效能与反应路径[J]. 生态环境学报, 2023, 32(11): 1933-1941. |
| [5] | 张睿含, 智燕彩, 贾明昊, 李晓娜, 王震宇. 生物质废弃物类型和水热pH对人工腐殖酸性能影响[J]. 生态环境学报, 2023, 32(8): 1496-1506. |
| [6] | 童永杰, 汪毅, 华玉妹, 赵建伟, 刘广龙, 蒋永参. 有机电子供体影响下硝酸盐和铁对磷转化的驱动作用[J]. 生态环境学报, 2023, 32(7): 1263-1274. |
| [7] | 王丽华, 王磊, 许端平, 薛杨. 煤胶体对重金属铜与镉的吸附特征研究[J]. 生态环境学报, 2023, 32(7): 1293-1300. |
| [8] | 闫学军, 郝赛梅, 张荣荣, 秦华, 高素莲, 王锋, 靳宪忠, 孙友敏, 张桂芹. 家居市场挥发性有机物排放成分谱及排放估算[J]. 生态环境学报, 2023, 32(6): 1070-1077. |
| [9] | 何贝贝, 范珊珊, 洪念, 刘安. 不同储存方式下屋顶雨水水质特性变化规律研究[J]. 生态环境学报, 2023, 32(3): 567-578. |
| [10] | 秦秦, 段海芹, 宋科, 孙丽娟, 孙雅菲, 周斌, 薛永. 常规施肥对土壤水稳性团聚体镉吸附解吸特性及化学形态的影响研究[J]. 生态环境学报, 2022, 31(12): 2403-2413. |
| [11] | 蒙素仟, 刘博, 赵嘉辉, 张傲, 赖华杰. 阳离子固相萃取填料微波快速制备及其在胺类有机污染物萃取中的应用[J]. 生态环境学报, 2022, 31(11): 2161-2168. |
| [12] | 姜晶, 阮呈杰, 陈霄宇, 吴仪, 汪永创. 微塑料模拟老化及其对污染物吸附行为影响研究进展[J]. 生态环境学报, 2022, 31(11): 2263-2274. |
| [13] | 姜晶, 邓精灵, 盛光遥. 生物炭老化及其对重金属吸附影响研究进展[J]. 生态环境学报, 2022, 31(10): 2089-2100. |
| [14] | 雷雅杰, 李雪, 常春艳, 毛雪飞. 聚苯乙烯微塑料对水中汞离子的吸附研究[J]. 生态环境学报, 2022, 31(10): 2048-2057. |
| [15] | 陈浩, 张玉莹, 钟妍, 张世伟, 陈俊伟, 冯加良. 上海市亚微米颗粒物中有机胺的浓度与组成特征[J]. 生态环境学报, 2022, 31(10): 2019-2027. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
