生态环境学报 ›› 2022, Vol. 31 ›› Issue (7): 1448-1455.DOI: 10.16258/j.cnki.1674-5906.2022.07.018
董乐恒1,2( ), 王旭刚1,*(
), 王旭刚1,*( ), 陈曼佳2,*(
), 陈曼佳2,*( ), 王子豪1, 孙丽蓉1, 石兆勇1, 吴琪琪2
), 王子豪1, 孙丽蓉1, 石兆勇1, 吴琪琪2
收稿日期:2022-03-03
出版日期:2022-07-18
发布日期:2022-08-31
通讯作者:
mjchen@soil.gd.cn作者简介:董乐恒(1997年生),男,硕士,主要从事土壤化学方面研究,E-mail: donglh1015@163.com
基金资助:
DONG Leheng1,2( ), WANG Xugang1,*(
), WANG Xugang1,*( ), CHEN Manjia2,*(
), CHEN Manjia2,*( ), WANG Zihao1, SUN Lirong1, SHI Zhaoyong1, Wu Qiqi2
), WANG Zihao1, SUN Lirong1, SHI Zhaoyong1, Wu Qiqi2
Received:2022-03-03
Online:2022-07-18
Published:2022-08-31
摘要:
厌氧条件下土壤中Fe氧化还原过程与重金属的环境行为密切相关。目前关于石灰性水稻土中Fe氧化还原过程中重金属Cu活性变化仍缺乏系统性研究。采用室内淹水厌氧培养方法,在模拟不同Cu污染程度的基础上,研究光照/避光条件下,石灰性水稻土中Fe氧化还原、Cu活性变化及体系中C转化动力学。结果表明,避光条件下,石灰性水稻土Fe(Ⅲ) 主要表现为还原过程,且随着Cu污染程度的加深,Fe最大还原速率逐渐降低,轻、中、重度Cu污染土壤分别降低了12.28%、17.54%、31.58%,Fe(Ⅲ) 的最大还原速率与Cu的污染程度呈显著负相关关系。同时,Fe(Ⅲ) 的还原过程能够降低土壤Cu活性,促进Cu形态转化,体系中0.5 mol∙L-1 HCl和DTPA提取态Cu含量分别下降了99.6%和96.1%;以中度污染土壤为例,避光培养结束后,体系未检测到弱酸提取态Cu,可还原态和可氧化态Cu的含量分别增加了30.5倍和25.1倍。光照条件下,体系中Fe的氧化还原表现为先Fe(Ⅲ) 还原、后Fe(Ⅱ) 氧化的过程,随着Cu污染程度的加深,对Fe(Ⅱ) 氧化的抑制作用逐渐增强,铁氧化还原菌是介导土壤Fe氧化还原过程的主要驱动力;同时,Fe氧化还原亦能影响土壤中Cu的活性,体系中0.5 mol∙L-1 HCl和DTPA提取态Cu含量分别下降了23.0%和74.5%;培养结束后,中度污染的石灰性水稻土中弱酸提取态Cu含量下降了94.5%,而可还原态和可氧化态Cu的含量分别增加了28.9倍和18.3倍。水溶性无机碳(WSIC)增加量与Cu活性降低量之间存在极显著正相关关系。以上研究结果可为石灰性土壤中Fe的氧化还原过程及重金属环境行为提供一定的理论依据。
中图分类号:
董乐恒, 王旭刚, 陈曼佳, 王子豪, 孙丽蓉, 石兆勇, 吴琪琪. 光照和避光条件下石灰性水稻土Fe氧化还原与Cu活性关系研究[J]. 生态环境学报, 2022, 31(7): 1448-1455.
DONG Leheng, WANG Xugang, CHEN Manjia, WANG Zihao, SUN Lirong, SHI Zhaoyong, Wu Qiqi. Interaction of Iron Redox and Cu Activities in Calcareous Paddy Soil under Light and Dark Condition[J]. Ecology and Environment, 2022, 31(7): 1448-1455.
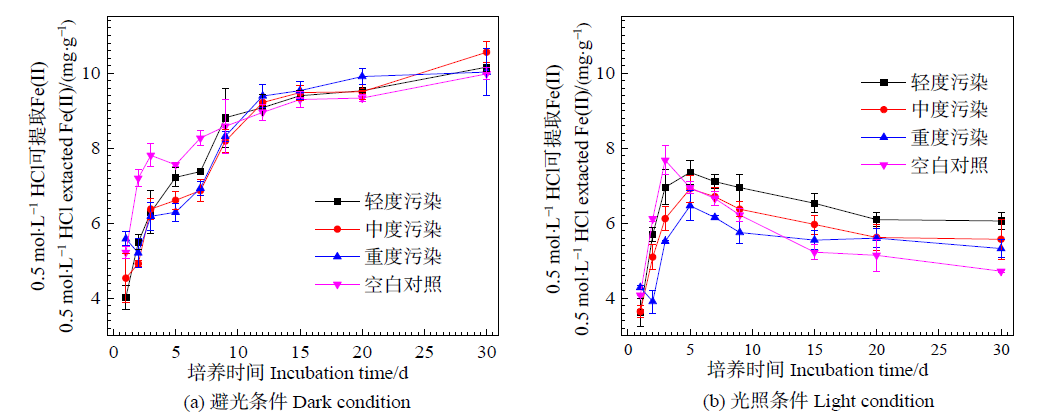
图1 避光(a)和光照(b)条件下0.5 mol∙L-1可浸提态Fe(Ⅱ)含量的变化
Figure 1 Change of 0.5 mol∙L-1 extracted Fe(II) concentration during incubation under dark (a) and light (b) conditions
| 处理 Treatment | 铁还原容量 Fe reduction capacity/(mg∙g-1) | 速率常数 Rate constant/d-1 | 铁最大还原速率 Max iron reduction rate/(mg∙g-1∙d-1) | 决定系数 r2 | 统计学概率 P |
|---|---|---|---|---|---|
| 空白对照 Control | 9.49±0.22 | 0.24±0.05 | 0.57±0.16a | 0.91 | <0.01 |
| 轻度污染 Light polluted | 9.96±0.15 | 0.20±0.03 | 0.50±0.09b | 0.98 | <0.01 |
| 中度污染 Moderate polluted | 9.86±0.28 | 0.19±0.04 | 0.47±0.08bc | 0.99 | <0.01 |
| 重度污染 Hight polluted | 10.45±0.52 | 0.15±0.03 | 0.39±0.06d | 0.93 | <0.01 |
表1 避光培养铁还原过程Logistic方程拟合参数
Table 1 Fitting parameters of logistic equation for iron reduction under dark condition
| 处理 Treatment | 铁还原容量 Fe reduction capacity/(mg∙g-1) | 速率常数 Rate constant/d-1 | 铁最大还原速率 Max iron reduction rate/(mg∙g-1∙d-1) | 决定系数 r2 | 统计学概率 P |
|---|---|---|---|---|---|
| 空白对照 Control | 9.49±0.22 | 0.24±0.05 | 0.57±0.16a | 0.91 | <0.01 |
| 轻度污染 Light polluted | 9.96±0.15 | 0.20±0.03 | 0.50±0.09b | 0.98 | <0.01 |
| 中度污染 Moderate polluted | 9.86±0.28 | 0.19±0.04 | 0.47±0.08bc | 0.99 | <0.01 |
| 重度污染 Hight polluted | 10.45±0.52 | 0.15±0.03 | 0.39±0.06d | 0.93 | <0.01 |
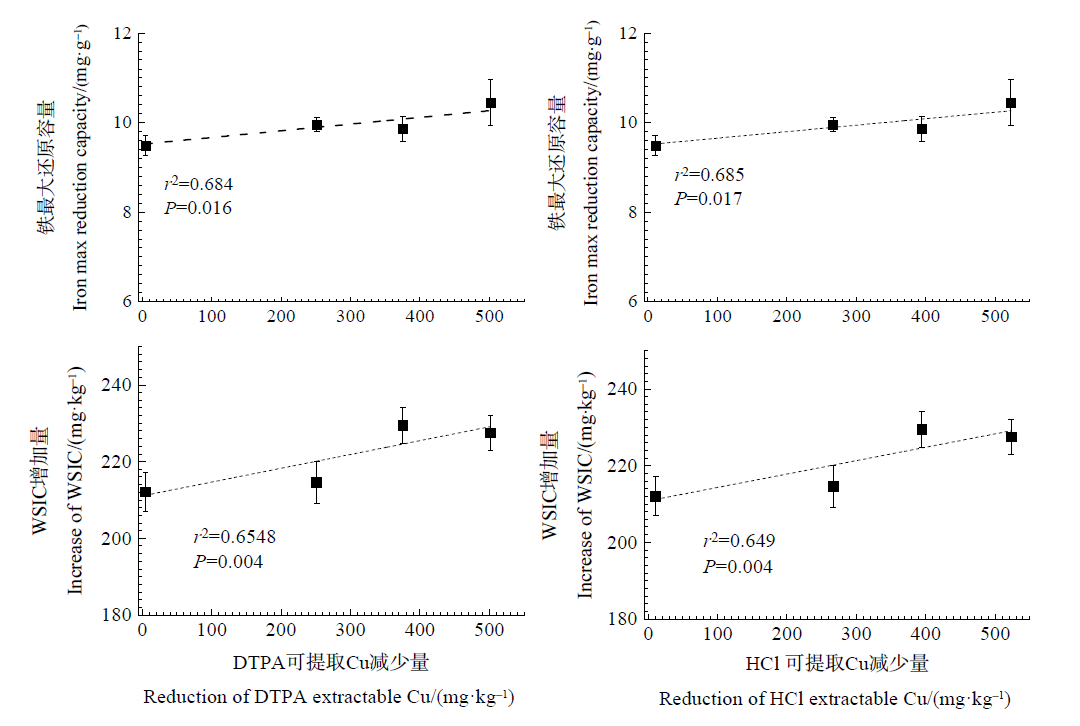
图5 Cu活性与铁最大还原容量和水溶性无机碳(WSIC)增加量相关性
Figure 5 Correlation between Cu activity and iron max reduction capacity and increase of water-soluble inorganic carbon (WSIC)
| [1] |
BALINT R, SAID-PULLICINO D, AJMONE-MARSAN F, 2015. Copper dynamics under alternating redox conditions is influenced by soil properties and contamination source[J]. Journal of Contaminant Hydrology, 173: 83-91.
DOI URL |
| [2] |
BINGJIE O, XIANCIA L, HUAN L, et al., 2014. Reduction of jarosite by shewanella oneidensis MR-1 and secondary mineralization[J]. Geochimica et Cosmochimica Acta, 124: 54-71.
DOI URL |
| [3] |
DAVRANCHE M, BOLINGER J, 2000. Heavy Metals Desorption from Synthesized and Natural Iron and Manganese Oxyhydroxides: Effect of Reductive Conditions[J]. Journal of Colloid and Interface Science, 227(2): 531-539.
DOI URL |
| [4] |
FAVARO M A, ROESCHLIN R A, RIBERO G G, et al., 2017. Relationships between copper content in orange leaves, bacterial biofilm formation and citrus canker disease control after different copper treatments[J]. Crop Protection, 92: 182-189.
DOI URL |
| [5] |
FRIERDICH A J, LUO Y, CATALANO J G, et al., 2011. Trace element cycling through iron oxide minerals during redox-driven dynamic recrystallization[J]. Geology (Boulder), 39(11): 1083-1086.
DOI URL |
| [6] |
KAPPLER A, 2005. Geomicrobiological cycling of iron[J]. Reviews in mineralogy and geochemistry, 59(1): 85-108.
DOI URL |
| [7] |
KUMPIENE J, LAGERKVIST A, MAURICE C, 2008. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments: A review[J]. Waste Management, 28(1): 215-225.
DOI URL |
| [8] |
LAMICHHANE J R, OSDAGHI E, BEHLAU F, et al., 2018. Thirteen decades of antimicrobial copper compounds applied in agriculture: A review[J]. Agronomy for sustainable development, 38(3): 1-18.
DOI URL |
| [9] |
LATTA D E, BACHMAN J E, SCHERER M M, 2012. Fe electron transfer and atom exchange in goethite: Influence of Al-substitution and anion sorption[J]. Environmental Science & Technology, 46(19): 10614-10623.
DOI URL |
| [10] |
LATTA D E, GORSKI C A, SCHERER M M, 2012. Influence of Fe2+- catalysed iron oxide recrystallization on metal cycling[J]. Biochemical Society Transactions, 40(6): 1191-1197.
DOI URL |
| [11] | LOVLEY D R, HOLMES D E, NEVIN K P, 2004. Dissimilatory Fe(III) and Mn(IV) Reduction[J]. Advances in Microbial Physiology, 55: 259-287. |
| [12] |
MAIZ I, ARAMBARRI I, GARCIA R, et al., 2000. Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis[J]. Environ Pollut, 110(1): 3-9.
DOI URL |
| [13] |
MATOCHA C J, KARATHANASIS A D, RAKSHIT S, et al., 2005. Reduction of copper(II) by iron(II)[J]. Journal of Environmental Quality, 34(5): 1539-1546.
DOI URL |
| [14] |
NEUBAUER S C, EMERSON D, MEGONIGAL J P, 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere[J]. Applied and Environmental Microbiology, 68(8): 3988-3995.
DOI URL |
| [15] |
OHADI S, GODAR A, MADSEN J, et al., 2021. Response of Rice Algal Assemblage to Fertilizer and Chemical Application: Implications for Early Algal Bloom Management[J]. Agronomy, 11(3): 542.
DOI URL |
| [16] |
RAURET G, LÓPEZ-SÁNCHEZ J F, SAHUQUILLO A, et al., 1999. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials[J]. Journal of Environmental Monitoring Jem, 1(1): 57-61.
DOI URL |
| [17] |
SANTANA LIMA J, 1994. Copper balances in cocoa agrarian ecosystems: effects of differential use of cupric fungicides[J]. Agriculture, Ecosystems & Environment, 48(1): 19-25.
DOI URL |
| [18] |
SCHNELL S, SCHINK B, WIDDEL F, et al., 1993. Ferrous iron oxidation by anoxygenic phototrophic bacteria[J]. Nature (London), 362(6423): 834-836.
DOI URL |
| [19] |
SHEN J, LI R, ZHANG F, et al., 2004. Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil[J]. Field Crops Research, 86(2-3): 225-238.
DOI URL |
| [20] |
TAO L, ZHU Z K, LI F B, et al., 2017. Fe(II)/Cu(II) interaction on goethite stimulated by an iron-reducing bacteria aeromonas hydrophila HS01 under anaerobic conditions[J]. Chemosphere, 187: 43-51.
DOI URL |
| [21] |
TAVARES-DIAS M, 2021. Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture[J]. Aquaculture, DOI: 10.1016/j.aquaculture.2021.736350.
DOI |
| [22] |
TESSIER A, FORTIN D, BELZILE N, et al., 1996. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurements[J]. Geochimica et Cosmochimica Acta, 60(3): 387-404.
DOI URL |
| [23] |
WANG X G, SUN L R, CHEN Z H, et al., 2020. Light inhibition of carbon mineralization associated with iron redox processes in calcareous paddy soil[J]. Journal of Soils and Sediments, 20(8): 3171-3180.
DOI URL |
| [24] |
WEBER K A, ACHENBACH L A, COATES J D, 2006. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction[J]. Nature Reviews Microbiology, 4(10): 752-764.
DOI URL |
| [25] | WU L S, ZENG D M, MO X R, et al., 2015. Immobilization impact of different fixatives on heavy metals contaminated soil[J]. Environmental Science, 36(1): 309-313. |
| [26] | 郝汉舟, 靳孟贵, 李瑞敏, 等, 2010. 耕地土壤铜、镉、锌形态及生物有效性研究[J]. 生态环境学报, 19(1): 92-96. |
| HAO H Z, JIN M G, LI R M, et al., 2010. Fractionations and bioavailability of Cu, Cd and Zn in cultivated land[J]. Ecology and Environmental Sciences, 19(1): 92-96. | |
| [27] | 刘勇, 刘燕, 朱光旭, 等, 2019. 石灰对Cu、Cd、Pb、Zn复合污染土壤中重金属化学形态的影响[J]. 环境工程, 37(2): 158-164. |
| LIU Y, LIU Y, ZHU G X, et al., 2019. Effects of lime on chemical forms of heavy metals under combined pollution of Cu, Cd, Pb and Zn in soil[J]. Environmental Engineering, 37(2): 158-164. | |
| [28] | 鲁如坤, 1999. 土壤农业化学分析方法[M]. 北京: 中国农业科学技术出版社. |
| LU R K, 1999. Analytical methods for soil and agro-chemistry[M]. Beijing: China Agricultural Science and Technology Press. | |
| [29] | 马小兰, 丁琳洁, 董军, 等, 2012. 地下环境中铁氧化物生物异化还原耦合降解硝基苯的影响因素研究[J]. 生态环境学报, 21(6): 1109-1114. |
| MA X L, DING L J, DONG J, et al., 2012. Influences of coupled degradation of nitrobenzene by iron oxides bacterial dissimilatory reduction in groundwater[J]. Ecology and Environmental Sciences, 21(6): 1109-1114. | |
| [30] | 钱子妍, 吴川, 何璇, 等, 2021. 铁循环微生物对环境中重金属的影响研究进展[J]. 环境化学, 40(3): 834-850. |
| QIAN Z Y, WU C, HE X, et al., 2021. Study on the influence of redox cycling microorganisms on heavy metals in the environment[J]. Environmental Chemistry, 40(3): 834-850. | |
| [31] | 全国土壤污染状况调查公报(2014-04-17)[J]. 环境教育, 2014, 06: 8-10. |
| Report on the national general survey of soil contamination (2014-04-17)[J]. Environmental education, 2014, 06: 8-10. | |
| [32] | 孙丽蓉, 黄海霞, 王旭刚, 等, 2013. 褐土中铁的氧化还原与碳素转化[J]. 土壤学报, 50(3): 540-547. |
| SUN L R, HUANG H X, WANG X G, et al., 2013. Relationship between anaerobic redox of iron oxides and carbon transformation in cinnamon soil[J]. Acta pedologica sinica, 50(3): 540-547. | |
| [33] | 孙丽蓉, 曲东, 卫亚红, 2008. 光照对水稻土中铁氧化还原的影响[J]. 土壤学报, 45(4): 628-634. |
| SUN L R, QU D, WEI Y H, 2008. Effect of illumination on iron oxide reduction in anaerobic paddy soils[J]. Acta Pedologica Sinaca, 45(4): 628-634. | |
| [34] | 王春, 童辉, 华建, 等, 2020. 铬取代针铁矿异化铁还原过程及铬的环境行为研究[J]. 生态环境学报, 29(9): 1883-1889. |
| WANG C, TONG H, HUA J, et al., 2020. Dissimilatory reduction of Cr-substituted goethite and its effect on Cr behavior[J]. Ecology and Environmental Sciences, 29(9): 1883-1889. | |
| [35] | 王静, 田然, 周辉, 等, 2010. 铜污染胁迫条件下农田土壤酶活性及微生物多样性对大气CO2浓度升高的响应[J]. 农业环境科学学报, 29(9): 1706-1711. |
| WANG J, TIAIN R, ZHOU H, et al., 2010. Response of soil enzymes and microbial communities to elevated concentration of atmospheric CO2 under stress of Cu pollution[J]. Journal of Agro-Environment Science, 29(9): 1706-1711. | |
| [36] | 王秀丽, 徐建民, 谢正苗, 等, 2002. 重金属铜和锌污染对土壤环境质量生物学指标的影响[J]. 浙江大学学报 (农业与生命科学版), 28(2): 74-78. |
| WANG X L, XU J M, XIE Z M, et al., 2002. Effects of Cu and Zn contamination on soil biological indicators of environmental quality[J]. Journal of Zhejiang University (Agriculture & Life Sciences), 28(2): 74-78. | |
| [37] | 王旭刚, 孙丽蓉, 马林娟, 等, 2018. 黄河中下游湿地土壤铁还原氧化过程的温度敏感性[J]. 土壤学报, 55(2): 380-389. |
| WANG X G, SUN L R, MA L J, et al., 2018. Temperature Sensitivity of Iron Redox Processes in Wetland Soil in the Middle and Lower Reaches of the Yellow River[J]. Acta Pedologica Sinica, 55(2): 380-389. | |
| [38] | 吴春艳, 陈义, 闵航, 等, 2006. Cd2+和Cu2+对水稻土微生物及酶活性的影响[J]. 浙江农业科学 (3): 303-307. |
| WU C Y, CHEN Y, MIN H, et al., 2006. Effects of Cd2+ and Cu2+ on microbial and enzyme activities in paddy soil[J]. Journal of Zhejiang Agricultural Sciences (3): 303-307. | |
| [39] | 徐丽娜, 李忠佩, 车玉萍, 2009. 淹水厌氧条件下腐殖酸对红壤中铁异化还原过程的影响[J]. 环境科学, 30(1): 221-226. |
| XU L N, LI Z P, CHE Y P, 2009. Influences of humic acids on the dissimilatory iron reduction of red soil in anaerobic condition[J]. Environmental Science, 30(1): 221-226. | |
| [40] | 周会程, 姚玉娇, 梁婷, 等, 2020. 天祝不同退化梯度高寒草甸土壤重金属污染及风险评价[J]. 生态环境学报, 29(10): 2102-2109. |
| ZHOU H C, YAO Y J, LIANG T, et al., 2020. Risk of Heavy Metal Pollution in Soil of Alpine Meadow with Different Degradation Gradients in Tianzhu County[J]. Ecology and Environmental Sciences, 29(10): 2102-2109. | |
| [41] | 朱先强, 石林, 2017. 矿物质钝化剂对铅铜复合型污染土壤的修复作用[J]. 生态环境学报, 26(4): 708-713. |
| ZHU X Q, SHI L, 2017. Remediation Effects of Mineral Amendment on Pb and Cu Contaminated Soil[J]. Ecology and Environmental Sciences, 26(4): 708-713. |
| [1] | 杜丹丹, 高瑞忠, 房丽晶, 谢龙梅. 旱区盐湖盆地土壤重金属空间变异及对土壤理化因子的响应[J]. 生态环境学报, 2023, 32(6): 1123-1132. |
| [2] | 冯树娜, 吕家珑, 何海龙. KI淋洗对黄绵土汞污染的去除效果及土壤理化性状的影响[J]. 生态环境学报, 2023, 32(4): 776-783. |
| [3] | 陈敏毅, 朱航海, 佘伟铎, 尹光彩, 黄祖照, 杨巧玲. 珠三角某遗留造船厂场地土壤重金属人体健康风险评估及源解析[J]. 生态环境学报, 2023, 32(4): 794-804. |
| [4] | 肖洁芸, 周伟, 石佩琪. 土壤重金属含量高光谱反演[J]. 生态环境学报, 2023, 32(1): 175-182. |
| [5] | 黄宏, 郑欣芸, 李迎东, 赵旭, 俞锦辰, 汪振华. 大陈岛海域不同年龄褐菖鲉对重金属富集作用研究[J]. 生态环境学报, 2022, 31(9): 1885-1891. |
| [6] | 马闯, 王雨阳, 周通, 吴龙华. 污染土壤颗粒态有机质镉锌富集特征及其解吸行为研究[J]. 生态环境学报, 2022, 31(9): 1892-1900. |
| [7] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| [8] | 李莹, 张洲, 杨高明, 祖艳群, 李博, 陈建军. 湿地植物根系泌氧能力和根表铁膜与根系吸收重金属的关系[J]. 生态环境学报, 2022, 31(8): 1657-1666. |
| [9] | 罗松英, 李秋霞, 邱锦坤, 邓素炎, 李一锋, 陈碧珊. 南三岛土壤-红树植物系统中重金属形态特征及迁移转化规律[J]. 生态环境学报, 2022, 31(7): 1409-1416. |
| [10] | 彭红丽, 谭海霞, 王颖, 魏建梅, 冯阳. 不同种植模式下土壤重金属形态分布差异与生态风险评价[J]. 生态环境学报, 2022, 31(6): 1235-1243. |
| [11] | 黄敏, 赵晓峰, 梁荣祥, 王鹏忠, 戴安然, 何晓曼. 3种螯合剂对Cd、Cu复合污染土壤淋洗修复的对比研究[J]. 生态环境学报, 2022, 31(6): 1244-1252. |
| [12] | 朱立安, 张会化, 程炯, 李婷, 林梓, 李俊杰. 珠江三角洲林业用地土壤重金属潜在生态风险格局分析[J]. 生态环境学报, 2022, 31(6): 1253-1262. |
| [13] | 施建飞, 靳正忠, 周智彬, 王鑫. 额尔齐斯河流域典型尾矿库区周边土壤重金属污染评价[J]. 生态环境学报, 2022, 31(5): 1015-1023. |
| [14] | 钱学诗, 李勇, 钱壮壮, 葛晓敏, 唐罗忠. 北亚热带东部次生阔叶林降水过程中的镉、铅、砷含量变化[J]. 生态环境学报, 2022, 31(5): 979-989. |
| [15] | 杨贤房, 陈朝, 郑林, 万智巍, 陈永林, 王远东. 稀土矿区不同土地利用类型土壤细菌群落特征及网络分析[J]. 生态环境学报, 2022, 31(4): 793-801. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
