生态环境学报 ›› 2021, Vol. 30 ›› Issue (7): 1503-1512.DOI: 10.16258/j.cnki.1674-5906.2021.07.019
张苏明1,2( ), 张建强2,3, 周凯1,*(
), 张建强2,3, 周凯1,*( ), 陈志良2,3,*(
), 陈志良2,3,*( )
)
收稿日期:2021-04-19
出版日期:2021-07-18
发布日期:2021-10-09
通讯作者:
*周凯(1969年生),男,副教授,博士,研究方向为城市生态环境治理。E-mail: kzhou89@126.com;陈志良(1976年生),男,研究员,博士,研究方向为土壤地下水污染防治。E-mail: chenzhiliang@scies.org作者简介:张苏明(1995年生),女,硕士研究生,研究方向为景观生态恢复技术研究。E-mail: 861595800 @qq.com
基金资助:
ZHANG Suming1,2( ), ZHANG Jianqiang2,3, ZHOU Kai1,*(
), ZHANG Jianqiang2,3, ZHOU Kai1,*( ), CHEN Zhiliang2,3,*(
), CHEN Zhiliang2,3,*( )
)
Received:2021-04-19
Online:2021-07-18
Published:2021-10-09
摘要:
目前针对生物炭修复重金属污染的水体、土壤方面的研究虽然很多,但是对其吸附污染物的机制研究却较少。为了提高生物炭对砷的吸附能力,以农业废弃物椰壳为原料,在300 ℃下利用硫酸及硫酸铁制备铁基改性生物炭,采用SEM-EDS、FTIR、XRD及XPS等手段对椰壳生物炭(CSB)、硫酸改性生物炭(SCSB)以及铁基改性生物炭(SFCSB)表面结构与特征进行表征,通过pH值影响实验、等温吸附实验和动力学吸附实验对CSB、SCSB及SFCSB 3种生物炭吸附砷(As)的效果进行比较。结果表明,硫酸及硫酸铁共同改性使生物炭的比表面积增大了1.56倍,表面官能团新增亚甲基(-CH3)和羧基(-COO),SFCSB表面的Fe吸附As(Ⅴ)后在Fe2p能级生成了Fe2O3和FeOOH,证明铁基改性成功。SFCSB对As(Ⅴ)的吸附符合Elovich动力学模型及Langmiur等温吸附模型,当pH=5时,SFCSB对砷的最大吸附量为14.65 mg∙g-1,与未改性的CSB相比吸附量提高了238倍。SFCSB对As(Ⅴ)的吸附方式为物理化学吸附,吸附机制包括生物炭表面正电荷与阴离子之间的静电吸引、O-H-As氢键结合、砷氧阴离子与铁氧化物的配位体效应和表面羟基官能团络合等。研究表明,铁基改性椰壳生物炭是一种高效的除砷吸附剂。该研究从农业废弃物利用和环境修复的角度出发,为制备更高效、能深度净化污染的生物炭提供参考,也为吸附机制的探讨提供理论依据。
中图分类号:
张苏明, 张建强, 周凯, 陈志良. 铁基改性椰壳生物炭对砷的吸附效果及机制研究[J]. 生态环境学报, 2021, 30(7): 1503-1512.
ZHANG Suming, ZHANG Jianqiang, ZHOU Kai, CHEN Zhiliang. Adsorption Effect and Mechanism of Iron-based Modified Coconut Shell Biochar to Arsenic[J]. Ecology and Environment, 2021, 30(7): 1503-1512.
| 样品 The sample | pH | 比表面积 Specific surface area/(m2∙g-1) | w(Fe)/ (mg∙g-1) |
|---|---|---|---|
| CSB | 9.92 | 247.382 | 1.78 |
| SCSB | 2.16 | 297.483 | 1.85 |
| SFCSB-10 | 1.95 | 387.224 | 3.03 |
表1 生物炭的基本性质
Table 1 Basic properties of biochar
| 样品 The sample | pH | 比表面积 Specific surface area/(m2∙g-1) | w(Fe)/ (mg∙g-1) |
|---|---|---|---|
| CSB | 9.92 | 247.382 | 1.78 |
| SCSB | 2.16 | 297.483 | 1.85 |
| SFCSB-10 | 1.95 | 387.224 | 3.03 |
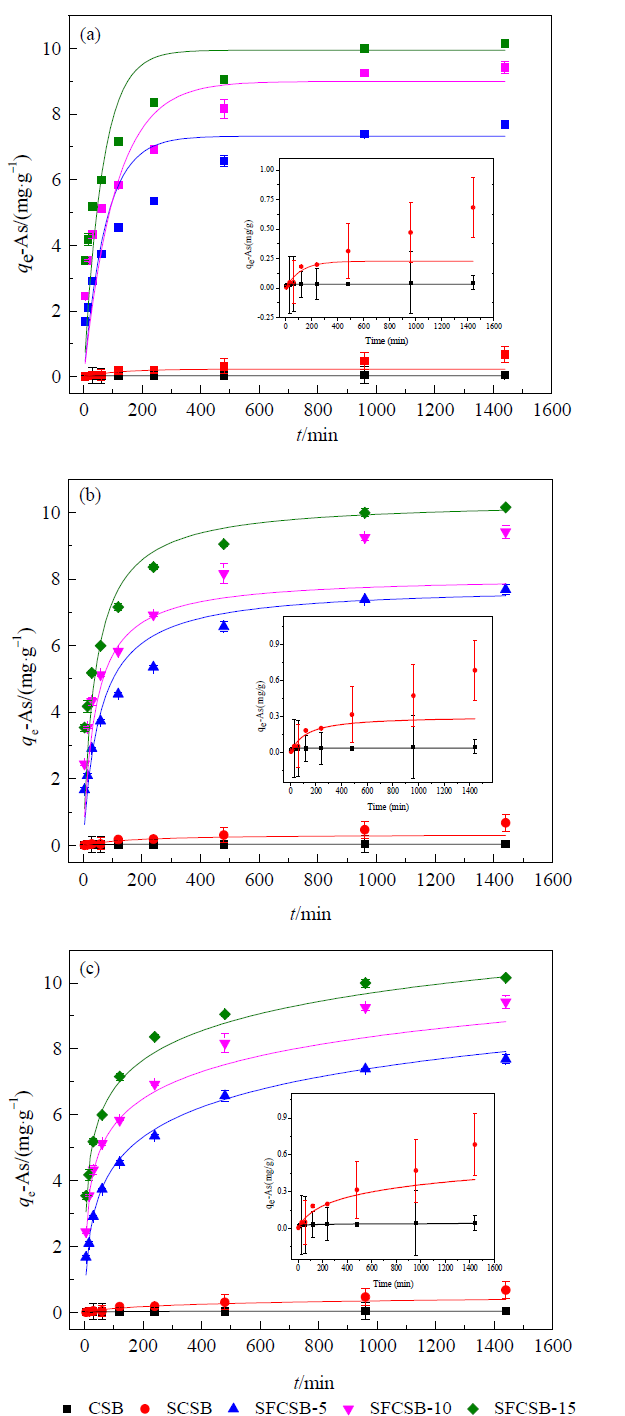
图7 生物炭对As(Ⅴ)的吸附动力学曲线 (a)准一级动力学模型Quasi first order dynamics,(b)准二级动力学模型Quasi second order dynamics,(c)Elovich动力学模型Elovich model
Fig. 7 Adsorption kinetic curve of As(Ⅴ) on biochar
| 样品 The sample | 准一级动力学 Quasi first order dynamics | 准二级动力学 Quasi second order dynamics | Elovich模型 Elovich model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe/(mg∙g-1) | k1/(min-1) | R2 | qe/(mg∙g-1) | k2/(g∙mg-1∙min-1) | R2 | α/(g∙mg-1∙min-1) | Β/(g∙mg-1) | R2 | |||
| CSB | 0.034 | 0.161 | 0.883 | 0.036 | 6.408 | 0.959 | 0.111 | 237.665 | 0.900 | ||
| SCSB | 0.228 | 0.009 | 0.993 | 0.340 | 0.018 | 0.992 | 0.001 | 2.412 | 0.975 | ||
| SFCSB-5 | 7.331 | 0.013 | 0.936 | 7.798 | 0.002 | 0.975 | 0.418 | 0.796 | 0.990 | ||
| SFCSB-10 | 5.926 | 0.063 | 0.767 | 8.088 | 0.003 | 0.855 | 1.110 | 0.747 | 0.992 | ||
| SFCSB-15 | 9.950 | 0.016 | 0.882 | 10.363 | 0.002 | 0.950 | 2.628 | 0.768 | 0.988 | ||
表2 动力学吸附As(Ⅴ)拟合参数
Table 2 Kinetic adsorption of As(Ⅴ) fitting parameters
| 样品 The sample | 准一级动力学 Quasi first order dynamics | 准二级动力学 Quasi second order dynamics | Elovich模型 Elovich model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe/(mg∙g-1) | k1/(min-1) | R2 | qe/(mg∙g-1) | k2/(g∙mg-1∙min-1) | R2 | α/(g∙mg-1∙min-1) | Β/(g∙mg-1) | R2 | |||
| CSB | 0.034 | 0.161 | 0.883 | 0.036 | 6.408 | 0.959 | 0.111 | 237.665 | 0.900 | ||
| SCSB | 0.228 | 0.009 | 0.993 | 0.340 | 0.018 | 0.992 | 0.001 | 2.412 | 0.975 | ||
| SFCSB-5 | 7.331 | 0.013 | 0.936 | 7.798 | 0.002 | 0.975 | 0.418 | 0.796 | 0.990 | ||
| SFCSB-10 | 5.926 | 0.063 | 0.767 | 8.088 | 0.003 | 0.855 | 1.110 | 0.747 | 0.992 | ||
| SFCSB-15 | 9.950 | 0.016 | 0.882 | 10.363 | 0.002 | 0.950 | 2.628 | 0.768 | 0.988 | ||
| 样品 The sample | Langmiur模型 Langmiur model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm/ (mg∙g-1) | KL/ (L∙mg-1) | R2 | KF/ (mg1-n∙g-1∙L-n) | $\frac{1}{n}/$ (g∙L-1) | R2 | ||
| CSB | 0.062 | 3.452 | 1.000 | 0.004 | 0.529 | 0.465 | |
| SCSB | 5.209 | 0.001 | 0.965 | 0.003 | 1.066 | 0.818 | |
| SFCSB-5 | 9.164 | 0.393 | 0.966 | 3.964 | 0.177 | 0.728 | |
| SFCSB-10 | 10.262 | 0.914 | 0.954 | 4.973 | 0.163 | 0.979 | |
| SFCSB-15 | 14.297 | 2.707 | 0.977 | 7.320 | 0.158 | 0.600 | |
表3 改性前后生物炭对As(Ⅴ)吸附等温线模型的拟合参数
Table 3 Fitting parameters of As(Ⅴ)-adsorption isotherm model of biochar before and after modification
| 样品 The sample | Langmiur模型 Langmiur model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm/ (mg∙g-1) | KL/ (L∙mg-1) | R2 | KF/ (mg1-n∙g-1∙L-n) | $\frac{1}{n}/$ (g∙L-1) | R2 | ||
| CSB | 0.062 | 3.452 | 1.000 | 0.004 | 0.529 | 0.465 | |
| SCSB | 5.209 | 0.001 | 0.965 | 0.003 | 1.066 | 0.818 | |
| SFCSB-5 | 9.164 | 0.393 | 0.966 | 3.964 | 0.177 | 0.728 | |
| SFCSB-10 | 10.262 | 0.914 | 0.954 | 4.973 | 0.163 | 0.979 | |
| SFCSB-15 | 14.297 | 2.707 | 0.977 | 7.320 | 0.158 | 0.600 | |
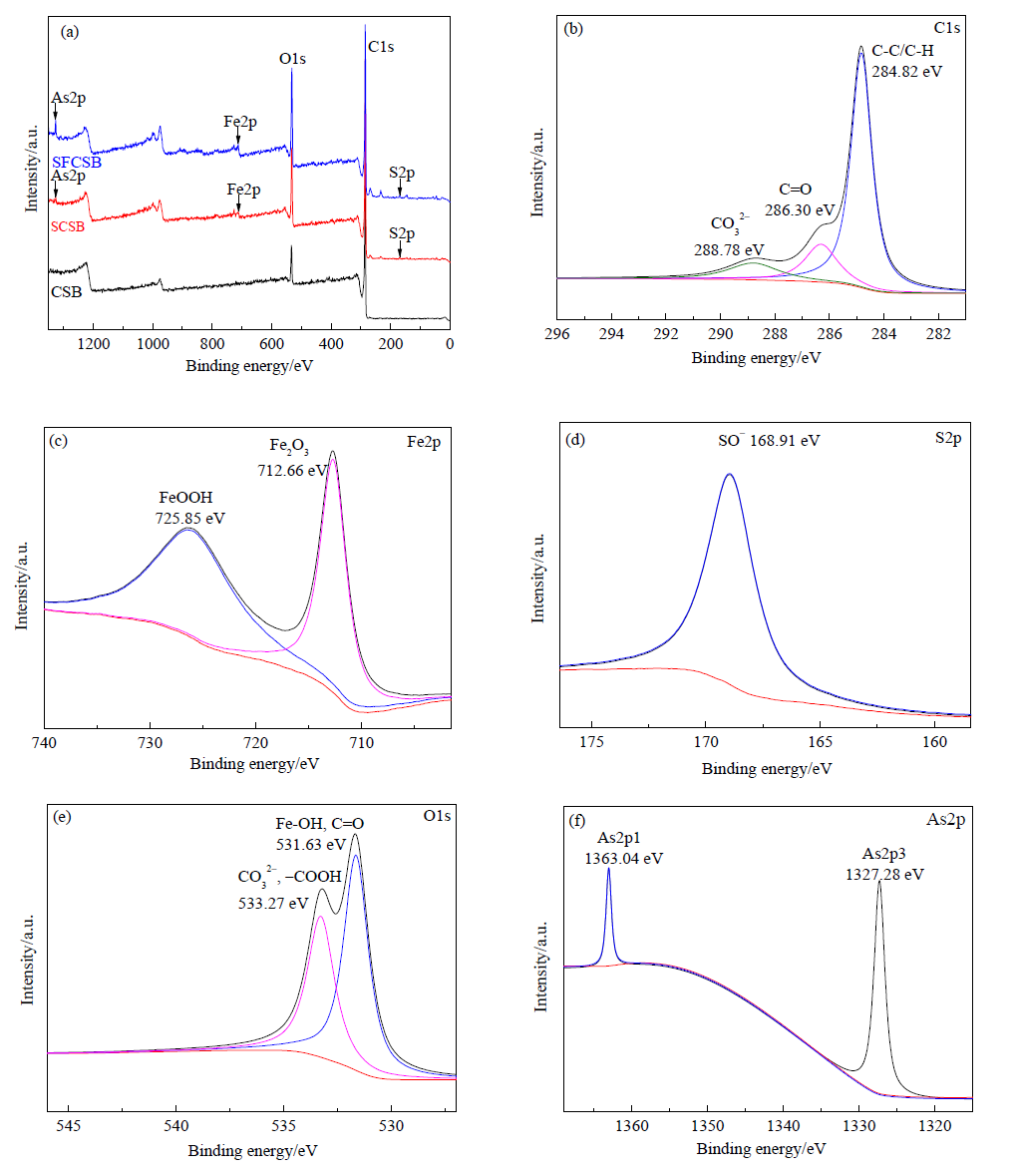
图10 SFCSB-10吸附As(Ⅴ)后的XPS(C1s、Fe2p、S2p、O1s、As2p)射线图谱 (b)C1s分峰图谱,(c)Fe2p分峰图谱,(d)S2p分峰图谱,(e)O1s分峰图谱,(f)As2p分峰图谱
Fig. 10 XPS (C1s, Fe2p, S2p, O1s, AS2p) spectrum of SFCSB-10 after adsorption of As(Ⅴ) (b) C1s peak segmentation map, (c) Fe2p peak segmentation map, (d) S2p peak segmentation map, (e) O1s peak segmentation map, (f) AS2p peak segmentation map
| [1] |
ANDERSON M A, FERGUSON J F, GAVIS J, 1976. Arsenate adsorption on amorphous aluminum hydroxide[J]. Journal of Colloid and Interface Science, 54(3): 391-399.
DOI URL |
| [2] |
BULUT E, ÖZACAR M, ŞENGIL İ A, 2008. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design[J]. Microporous and Mesoporous Materials, 115(3): 234-246.
DOI URL |
| [3] |
CHANG J N, ZHANG H B, CHENG H Y, et al., 2020. Spent Ganoderma lucidum substrate derived biochar as a new bio-adsorbent for Pb2+/Cd2+ removal in water[J]. Chemosphere, DOI: 10.1016/j.chemosphere.2019. 125121.
DOI |
| [4] | CHEN H M, 2005. Environmental Soil Science[M]. Beijing: Science Press. |
| [5] |
DIXIT S, HERING J G, 2003. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility[J]. Environmental Science and Technology, 37(18): 4182-4189.
DOI URL |
| [6] |
DIXIT S, HERING J G, 2006. Sorption of Fe(II) and As(III) on goethite in single- and dual-sorbate systems[J]. Chemical Geology, 228(1-3): 6-15.
DOI URL |
| [7] |
HU W Y, ZHANG Y X, HUANG B, et al., 2017. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies[J]. Chemosphere, 170: 183-195.
DOI URL |
| [8] |
HYUN M J, EUNSUNG K, 2019. Engineered biochar from agricultural waste for removal of tetracycline in water[J]. Bioresource Technology, 284: 437-447.
DOI URL |
| [9] |
JUNG K W, HWANG M J, JEONG T U, et al., 2015. A novel approach for preparation of modified-biochar derived from marine macroalgae: Dual purpose electro-modification for improvement of surface area and metal impregnation[J]. Bioresource Technology, 191: 342-345.
DOI URL |
| [10] |
KEILUWEIT M, NICO P S, JOHNSON M G, et al., 2010. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science and Technology, 44(4): 1247-1253.
DOI URL |
| [11] |
PARK J H, WANG J J, KIM S H, et al., 2019. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures[J]. Journal of Colloid and Interface Science, 553: 298-307.
DOI URL |
| [12] |
PENG H B, GAO P, CHU G, et al., 2017. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars[J]. Environmental Pollution, 229: 846-853.
DOI URL |
| [13] |
BOYLE R W, JONASSON I R, 1973. The geochemistry of arsenic and its use as an indicator element in geochemical prospecting[J]. Journal of Geochemical Exploration, 2(3): 251-296.
DOI URL |
| [14] |
GUO Y, HUANG W, CHEN B, et al., 2017. Removal of tetracycline from aqueous solution by MCM-41-zeolite a loaded nano zero valent iron: Synthesis, characteristic, adsorption performance and mechanism[J]. Journal of Hazardous Materials, 339: 22-32.
DOI URL |
| [15] |
RAJAPAKSHA A U, CHEN S S, TSANG D C, et al., 2016. Engineered/ designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification[J]. Chemosphere, 148: 276-291.
DOI URL |
| [16] |
RATTANACHUESKUL N, SANING A, KAOWPHONG S, et al., 2017. Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process[J]. Bioresource Technology, 226: 164-172.
DOI URL |
| [17] |
SAJJADI B, ZUBATIUK T, LESZCZYNSKA D, et al., 2019. Chemical activation of biochar for energy and environmental applications: a comprehensive review[J]. Reviews in Chemical Engineering, 35(7): 777-815.
DOI URL |
| [18] | TAN Z, YUAN S, HONG M, et al., 2020. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd[J]. Journal of Hazardous Materials, 56: 597-606. |
| [19] |
WU J Z, HUANG D, LIU X M, et al., 2018. Remediation of As(Ⅲ) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar[J]. Journal of Hazardous Materials, 348: 10-19.
DOI URL |
| [20] | YANG A L, ZHU Y K, HUANG C P, 2018. Facile preparation and adsorption performance of graphene oxide-manganese oxide-manganese oxide composite for uranium[J]. Scientific Reports, 8(1): 1-10. |
| [21] | YANG D, WANG L, LI Z T, et al., 2020. Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems[J]. Science of the Total Environment, 708: 1-11. |
| [22] |
YUAN J H, XU R K, ZHANG H, 2011. The forms of alkalis in the biochar produced from crop residues at different temperatures[J]. Bioresource Technology, 102(3): 3488-3497.
DOI URL |
| [23] |
ZHANG L K, QIN X Q, TANG J S, et al., 2017. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China[J]. Applied Geochemistry, 77: 80-88.
DOI URL |
| [24] |
ZHANG S J, LI X Y, CHEN J P, 2020. An XPS study for mechanisms of arsenate adsorption onto a magnetite-doped activated carbon fiber[J]. Journal of Colloid and Interface Science, 343(1): 232-238.
DOI URL |
| [25] | ZHOU Y Y, LIU X C, XIANG Y J, et al., 2017. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology, 245(Part A): 266-273. |
| [26] | 陈坦, 周泽宇, 孟瑞红, 等, 2019. 改性污泥基生物炭的性质与重金属吸附效果[J]. 环境科学, 40(4): 1842-1848. |
| CHEN T, ZHOU Z Y, MENG R H, et al., 2019. Characteristics and heavy metal adsorption performance of sewage sludge-derived biochar from co-pyrolysis with transition metals[J]. Environmental Science, 40(4): 1842-1848. | |
| [27] | 楚颖超, 李建宏, 吴蔚东, 2015. 椰纤维生物炭对Cd(Ⅱ)、As(Ⅲ)、Cr(Ⅲ)和Cr(Ⅵ)的吸附[J]. 环境工程学报, 9(5): 2165-2170. |
| CHU Y C, LI J H, WU W D, 2015. Adsorption of Cd(Ⅱ), As(Ⅲ), Cr(Ⅲ) and Cr(Ⅵ) by coconut fiber-derived biochars[J]. Chinese Journal of Environmental Engineering, 9(5): 2165-2170. | |
| [28] | 楚颖超, 2015. 不同温度裂解椰子生物炭对重金属吸附的研究[D]. 海口: 海南大学. |
| CHU Y C, 2015. Adsorption of heavy metal on different temperature derived biochars from coconut[D]. Haikou: Hainan University. | |
| [29] | 国家质量技术监督局, 1999. 木质活性炭试验方法pH值的测定: GB/T 12496.7—1999 [S]. 北京: 中国标准出版社. |
| The State Bureau of Quality and Technical Supervision, 1991. Test method for wooden activated carbon. Determination of pH: GB/T 12496.7—1999[S]. Beijing: China Standard Press. | |
| [30] | 罗海艳, 李丹阳, 刘寿涛, 等, 2019. 铁锰改性椰壳炭对土壤镉形态及水稻吸收积累镉的影响[J]. 环境科学研究, 32(5): 857-865. |
| LUO H Y, LI D Y, LIU S T, et al., 2019. Effects of Fe-Mn Modified Coconut Shell Carbon on Cadmium Formation in Soil and Cadmium Absorption and Accumulation in Rice[J]. Research of Environmental Sciences, 32(5): 857-865. | |
| [31] | 马洁晨, 汪新亮, 张学胜, 等, 2019. 不同热解温度龙虾壳生物炭特征及对Zn2+的吸附机制[J]. 生态与农村环境学报, 35(7): 900-908. |
| MA J C, WANG X L, ZHANG X S, et al., 2019. Influence of pyrolysis temperature on characteristics and Zn2+ adsorptive mechanism of crayfish shell biochars[J]. Journal of Ecology and Rural Environment, 35(7): 900-908. | |
| [32] | 平森文, 朱政, 盛又聪, 等, 2019. 生物炭去除土壤中重金属效果主要影响因素的研究进展[J]. 现代农业科技 (12): 153-155, 160. |
| PING S W, ZHU Z, SHENG Y C, et al., 2019. Research progress on the main influencing factors of the effect of biochar on removal of heavy metals in soil[J]. Modern Agricultural Technology (12): 153-155, 160. | |
| [33] | 邱家枝, 2016. 椰壳生物炭的制备及其应用研究[D]. 福州: 福州大学. |
| QIU J Z, 2016. Praparation and Application of coconut shell biochar[D]. Fuzhou: Fuzhou University. | |
| [34] | 阳昕, 2016. 铁活化污泥基吸附剂的制备及对水中污染物去除效能研究[D]. 哈尔滨: 哈尔滨工业大学. |
| YANG X, 2016. Preparation of ferric-activated sluge-based adsorbent and its efficiency on the removal of contaminants from wastewaters[D]. Harbin: Harbin Institute of Technology. | |
| [35] | 杨放, 李心清, 王兵, 等, 2015. 热解材料对生物炭理化性质的影响[J]. 农业环境科学学报, 34(9): 1822-1828. |
| YANG F, LI X Q, WANG B, et al., 2015. The effect of pyrolysis materials on the physical and chemical properties of biochar[J]. Journal of Agricultural Environmental Sciences, 34(9): 1822-1828. | |
| [36] | 杨居荣, 1986. 砷在土壤中的蓄积与迁移特征[J]. 环境科学, 7(2): 26-31. |
|
YANG J R, 1986. Arsenic accumulate and migrate characteristic in soils[J]. Environmental Science, 7(2): 26-31.
DOI URL |
|
| [37] | 赵天赐, 周世真, 马小龙, 等, 2019. 负载铁锰氧化物的玉米芯炭对Pb2+的吸附作用[J]. 环境科学学报, 39(9): 2997-3009. |
| ZHAO T C, ZHOU S Z, MA X L, et al., 2019. Study on the adsorption of Pb2+ by MnFeOX-loaded corncob biochar[J]. Journal of Environmental Sciences, 39(9): 2997-3009. | |
| [38] | 朱司航, 赵晶晶, 尹英杰, 等, 2019. 针铁矿改性生物炭对砷吸附性能[J]. 环境科学, 40(6): 2773-2782. |
| ZHU S H, ZHAO J J, YIN Y J, et al., 2019. Application of goethite modified biochar for arsenic removal from aqueous solution[J]. Environmental Science, 40(6): 2773-2782. |
| [1] | 黄英梅, 钟松雄, 朱忆雯, 王向琴, 李芳柏. 单质硫抑制水稻植株甲基汞累积的效应与机制[J]. 生态环境学报, 2023, 32(6): 1115-1122. |
| [2] | 袁林江, 李梦博, 冷钢, 钟冰冰, 夏大朋, 王景华. 厌氧环境下硫酸盐还原与氨氧化的协同作用[J]. 生态环境学报, 2023, 32(1): 207-214. |
| [3] | 王钊, 张曼胤, 胡宇坤, 刘魏魏, 张苗苗. 盐度对典型滨海湿地沉积物汞甲基化的影响[J]. 生态环境学报, 2022, 31(9): 1876-1884. |
| [4] | 邓晓, 武春媛, 杨桂生, 李怡, 李勤奋. 椰壳生物炭对海南滨海土壤的改良效果[J]. 生态环境学报, 2022, 31(4): 723-731. |
| [5] | 程文远, 李法云, 吕建华, 吝美霞, 王玮. 碱改性向日葵秸秆生物炭对多环芳烃菲吸附特性研究[J]. 生态环境学报, 2022, 31(4): 824-834. |
| [6] | 苏焱, 全妍红, 宦紫嫣, 姚佳, 苏小娟. 磷改性生物炭对云南某铅锌矿周边农田铅锌污染土壤修复效果的影响[J]. 生态环境学报, 2022, 31(3): 593-602. |
| [7] | 姜晶, 邓精灵, 盛光遥. 生物炭老化及其对重金属吸附影响研究进展[J]. 生态环境学报, 2022, 31(10): 2089-2100. |
| [8] | 童辉, 乔江涛, 周继梅, 雷琴凯, 陈曼佳, 刘承帅. 硫酸盐还原菌介导针铁矿表面硫的转化及镉固定脱毒效应[J]. 生态环境学报, 2021, 30(5): 1069-1075. |
| [9] | 王亚琢, 周翔, 修磊, 单锐, 袁浩然. 高铁酸钾改性生物炭的制备及其对水体中Cd(Ⅱ)的吸附特性[J]. 生态环境学报, 2021, 30(12): 2380-2386. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
