生态环境学报 ›› 2022, Vol. 31 ›› Issue (4): 824-834.DOI: 10.16258/j.cnki.1674-5906.2022.04.021
程文远1,2( ), 李法云2,3,*(
), 李法云2,3,*( ), 吕建华1,*, 吝美霞4, 王玮2,3
), 吕建华1,*, 吝美霞4, 王玮2,3
收稿日期:2020-12-11
出版日期:2022-04-18
发布日期:2022-06-22
通讯作者:
*李法云,男,教授,博士,博士研究生导师。E-mail: lnecology@163.com作者简介:程文远(1987年生),男,硕士研究生。E-mail: wenyuancheng2020@163.com
基金资助:
CHENG Wenyuan1,2( ), LI Fayun2,3,*(
), LI Fayun2,3,*( ), LÜ Jianhua1,*, LIN Meixia4, WANG Wei2,3
), LÜ Jianhua1,*, LIN Meixia4, WANG Wei2,3
Received:2020-12-11
Online:2022-04-18
Published:2022-06-22
摘要:
为了实现农业秸秆废弃物的资源化利用,加强对生态环境中多环芳烃污染的控制,选取农业废弃物向日葵(Helianthus annuus)秸秆为原料,在不同温度条件下(300、500、700 ℃)烧制生物炭(BC300、BC500、BC700),同时在500 ℃条件下制备KOH改性生物炭(A-BC500),采用元素分析仪、比表面积分析仪、扫描电子显微镜、X射线衍射仪和傅里叶红外光谱仪分别对其元素组成、比表面积、表观形貌、物相结构和官能团组成进行表征,并采用动力学吸附实验和等温吸附实验研究不同生物炭对多环芳烃菲的吸附性能。结果表明,炭化温度及碱改性均会影响生物炭的元素组成,进而改变其芳香性、亲水性和极性。向日葵秸秆生物炭的炭质骨架结构随着炭化温度升高而逐步发生变形和坍塌;与BC500相比,A-BC500的表面结构粗糙程度增加且比表面积增加至529.14 m2∙g-1。生物炭对菲的动力学吸附曲线符合准二级动力学模型(R2>0.99),较BC500、A-BC500对菲的平衡吸附量提高了12%,且准二级动力学吸附速率常数提高了约2.3倍,能较快达到菲的吸附平衡状态;等温吸附曲线中Freundlich模型(R2>0.90)和Langmuir模型(R2>0.90)均可用于描述生物炭对菲的吸附过程,且该过程较容易进行;溶液pH对生物炭吸附菲的影响较小;生物炭吸附菲前后的FTIR谱图显示,氢键和π-π相互作用对生物炭吸附菲具有一定的贡献;自由基猝灭实验证明,∙OH参与了生物炭吸附菲的过程。生物炭与多环芳烃之间的相互作用机制较为复杂,并非简单的单分子层物理吸附,还包括多环芳烃与生物炭表面的化学反应。
中图分类号:
程文远, 李法云, 吕建华, 吝美霞, 王玮. 碱改性向日葵秸秆生物炭对多环芳烃菲吸附特性研究[J]. 生态环境学报, 2022, 31(4): 824-834.
CHENG Wenyuan, LI Fayun, LÜ Jianhua, LIN Meixia, WANG Wei. Sorption Characteristics of Polycyclic Aromatic Hydrocarbons Phenanthrene on Sunflower Straw Biochar Modified with Alkali[J]. Ecology and Environment, 2022, 31(4): 824-834.
| 名称 Name | 分子结构 Molecular formula | 化学结构 Chemical structure | 分子质量 Molecular mass/ (g∙mol-1) | 辛醇-水分配系数 Octanol-water partition coefficients | 溶解度 Solubility/ (mg∙L-1) | 分子体积 Molecular volume/ nm3 |
|---|---|---|---|---|---|---|
| 菲 Phenanthrene | C14H10 | 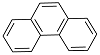 | 178.23 | 4.57 | 1.18 | 0.347 |
表1 菲的理化性质
Table 1 Physicochemical properties of phenanthrene
| 名称 Name | 分子结构 Molecular formula | 化学结构 Chemical structure | 分子质量 Molecular mass/ (g∙mol-1) | 辛醇-水分配系数 Octanol-water partition coefficients | 溶解度 Solubility/ (mg∙L-1) | 分子体积 Molecular volume/ nm3 |
|---|---|---|---|---|---|---|
| 菲 Phenanthrene | C14H10 |  | 178.23 | 4.57 | 1.18 | 0.347 |
| Sample | C, H, N, O contents/% | Atomic ratios | BET/ (m2∙g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | (N+O)/C | O/C | H/C | |||
| BC300 | 45.27 | 2.24 | 1.57 | 27.82 | 0.65 | 0.61 | 0.05 | 19.72 | |
| BC500 | 51.69 | 1.35 | 1.62 | 16.16 | 0.34 | 0.31 | 0.03 | 93.26 | |
| BC700 | 55.30 | 0.66 | 1.71 | 10.45 | 0.22 | 0.19 | 0.01 | 523.57 | |
| A-BC500 | 42.71 | 0.73 | 1.26 | 19.51 | 0.49 | 0.46 | 0.02 | 529.14 | |
表2 生物炭的元素组成与原子比
Table 2 Elemental compositions and atomic ratios of biochars
| Sample | C, H, N, O contents/% | Atomic ratios | BET/ (m2∙g-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | (N+O)/C | O/C | H/C | |||
| BC300 | 45.27 | 2.24 | 1.57 | 27.82 | 0.65 | 0.61 | 0.05 | 19.72 | |
| BC500 | 51.69 | 1.35 | 1.62 | 16.16 | 0.34 | 0.31 | 0.03 | 93.26 | |
| BC700 | 55.30 | 0.66 | 1.71 | 10.45 | 0.22 | 0.19 | 0.01 | 523.57 | |
| A-BC500 | 42.71 | 0.73 | 1.26 | 19.51 | 0.49 | 0.46 | 0.02 | 529.14 | |
| Sample | qe | Pseudo-first Order Kinetic Model | Pseudo-second Order Kinetic Model | Intra-particle diffusion model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe, 1 | k1 | R2 | qe, 2 | k2 | R2 | qe, id | kid | C | R2 | ||||
| BC300 | 3.71 | 3.39 | 0.53 | 0.9319 | 3.78 | 0.19 | 0.9990 | 3.87 | 0.46 | 1.62 | 0.8104 | ||
| BC500 | 4.25 | 3.94 | 0.77 | 0.9073 | 4.27 | 0.27 | 0.9986 | 4.43 | 0.41 | 2.43 | 0.7540 | ||
| BC700 | 5.19 | 5.06 | 2.14 | 0.8547 | 5.17 | 1.34 | 0.9938 | 5.25 | 0.13 | 4.62 | 0.6147 | ||
| A-BC500 | 4.79 | 4.63 | 1.72 | 0.8177 | 4.78 | 0.89 | 0.9977 | 4.87 | 0.18 | 3.99 | 0.6657 | ||
表3 生物炭对菲的吸附动力学拟合参数
Table 3 Fitting parameters of the sorption kinetics of phenanthrene by biochars
| Sample | qe | Pseudo-first Order Kinetic Model | Pseudo-second Order Kinetic Model | Intra-particle diffusion model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe, 1 | k1 | R2 | qe, 2 | k2 | R2 | qe, id | kid | C | R2 | ||||
| BC300 | 3.71 | 3.39 | 0.53 | 0.9319 | 3.78 | 0.19 | 0.9990 | 3.87 | 0.46 | 1.62 | 0.8104 | ||
| BC500 | 4.25 | 3.94 | 0.77 | 0.9073 | 4.27 | 0.27 | 0.9986 | 4.43 | 0.41 | 2.43 | 0.7540 | ||
| BC700 | 5.19 | 5.06 | 2.14 | 0.8547 | 5.17 | 1.34 | 0.9938 | 5.25 | 0.13 | 4.62 | 0.6147 | ||
| A-BC500 | 4.79 | 4.63 | 1.72 | 0.8177 | 4.78 | 0.89 | 0.9977 | 4.87 | 0.18 | 3.99 | 0.6657 | ||
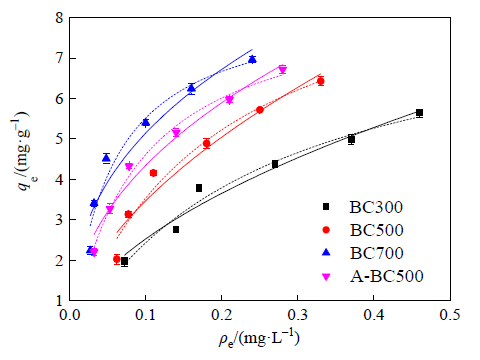
图7 生物炭对菲的等温吸附曲线及拟合曲线 实线为Freundlich模型,虚线为Langmuir模型
Figure 7 Sorption isotherms and fitted curves of phenanthrene by biochars The solid line is Freundlich model, and the dotted line is Langmuir model
| Sample | Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm | kl | R2 | kf | n | R2 | ||
| BC300 | 8.60 | 3.95 | 0.97 | 8.60 | 0.53 | 0.96 | |
| BC500 | 9.98 | 5.50 | 0.96 | 12.00 | 0.54 | 0.93 | |
| BC700 | 8.43 | 18.98 | 0.94 | 12.50 | 0.39 | 0.90 | |
| A-BC500 | 8.62 | 11.54 | 0.99 | 11.97 | 0.44 | 0.96 | |
表4 生物炭对菲的吸附等温线拟合参数
Table 4 Fitting parameters of the sorption isotherms of phenanthrene by biochars
| Sample | Langmuir model | Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| qm | kl | R2 | kf | n | R2 | ||
| BC300 | 8.60 | 3.95 | 0.97 | 8.60 | 0.53 | 0.96 | |
| BC500 | 9.98 | 5.50 | 0.96 | 12.00 | 0.54 | 0.93 | |
| BC700 | 8.43 | 18.98 | 0.94 | 12.50 | 0.39 | 0.90 | |
| A-BC500 | 8.62 | 11.54 | 0.99 | 11.97 | 0.44 | 0.96 | |
| [1] |
ALI N, IMI I, KHDER M, et al., 2017. Polycyclic aromatic hydrocarbons (PAHs) in the settled dust of automobile workshops, health and carcinogenic risk evaluation[J]. Science of the Total Environment, 601-602: 478-484.
DOI URL |
| [2] |
CAGNON B, PY X, GUILLOT A, et al., 2009. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors[J]. Bioresource Technology, 100(1): 292-298.
DOI URL |
| [3] |
CHEN B L, JOHNSON E J, CHEFETZ B, et al., 2005. Sorption of polar andnonpolar aromatic organic contaminants by plant cuticular materials: The role of polarity andaccessibility[J]. Environmental Science & Technology, 39(16): 6138-6146.
DOI URL |
| [4] |
CHEN B L, ZHOU D D, ZHU L Z, 2008. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science and Technology, 42(14): 5137-5143.
DOI URL |
| [5] |
EDOKPAYI J, ODIYO J, POPOOLA O, et al., 2016. Determination and distribution of polycyclic aromatic hydrocarbons in rivers, sediments and wastewater effluents in Vhembe District, South Africa[J]. International Journal of Environmental Research and Public Health, 13(4): 387.
DOI URL |
| [6] |
El-ASHTOUKHY E S Z, AMIN N K, ABDELWAHAB O, 2008. Removal of lead (Ⅱ) and copper (Ⅱ) from aqueous solution using pomegranate peel as a new adsorbent[J]. Desalination, 223(1-3): 162-173.
DOI URL |
| [7] | ERTUGAY N, MALKOC E, 2014. Adsorption isotherm, kinetic, and thermodynamic studies for methylene blue from aqueous solution by needles of Pinus sylvestris L.[J]. Polish Journal of Environmental Studies, 23(6): 1995-2006. |
| [8] |
FLOTRON V, DELTEIL C, PADELLEC Y, et al., 2005. Removal of sorbed polycyclic aromatic hydrocarbons from soil, sludge and sediment samples using the Fenton’s reagent process[J]. Chemosphere, 59(10): 1427-1437.
DOI URL |
| [9] | GAO J, WANG Y, ZHOU S, et al., 2017. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance[J]. Chem Cat Chem, 9(9): 1708-1715. |
| [10] |
HOSSAIN M K, STREVOZ V, CHAN K Y, et al., 2011. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar[J]. Journal of Environmental Management, 92(1): 223-228.
DOI URL |
| [11] |
INYANG M, GAO B, PULLAMMANAPPALLIL P, et al., 2010. Biochar from anaerobically digested sugarcane bagasse[J]. Bioresource Technology, 101(22): 8868-8872.
DOI URL |
| [12] |
JIANG L, LIU Y, LIU S, et al., 2017. Adsorption of estrogen contaminants by graphene nanomaterials under natural organic matter preloading: comparison to carbon nanotube, biochar, and activated carbon[J]. Environmental Science & Technology, 51: 6352-6359.
DOI URL |
| [13] |
KEILUWEIT M, NICO P S, JOHNSON M G, et al., 2010. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science and Technology, 44(4): 1247-1253.
DOI URL |
| [14] |
KUBATOVA A, JANSEN B, VAUDOISOT J F, et al., 2002. Thermodynamic and kinetic models for the extraction of essential oil from savory and polycyclic aromatic hydrocarbons from soil with hot (subcritical) water and supercritical CO2[J]. Journal of Chromatography A, 975(1): 175-188.
DOI URL |
| [15] |
LI F Y, LIN M X, 2020. Synthesis of biochar-supported K-doped g-C3N4 photocatalyst for enhancing the polycyclic aromatic hydrocarbon degradation activity[J]. International Journal of Environmental Research and Public Health, 17(6): 2065.
DOI URL |
| [16] |
LIAN F, SUN B B, SONG Z G, et al., 2014. Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole[J]. Chemical Engineering Journal, 248: 128-134.
DOI URL |
| [17] |
LIOU T H, WU S J, 2009. Characteristics of microporous/mesoporous carbons prepared from rice husk under base and acid-treated conditions[J]. Journal of Hazardous Materials, 171(1-3): 693-703.
DOI URL |
| [18] | LYU H H, GAO B, HE F, et al., 2017. Ball-milled carbon nanomaterials for energy and environmental applications[J]. ACS Sustainable Chemistry & Engineering, 5(11): 9568-9585. |
| [19] |
LYU H H, ZHANG Q R, SHEN B X, et al., 2020. Application of biochar and its composites in catalysis[J]. Chemosphere, 240: 124842.
DOI URL |
| [20] |
MISHRA A K, AROCKIADOSS T, RAMAPRABHU S, 2010. Study of removal of azo dye by functionalized multi walled carbon nanotubes[J]. Chemical Engineering Journal, 162(3): 1026-1034.
DOI URL |
| [21] |
MOHAN D, SARSWAT A, Ok Y S, et al., 2014. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-A critical review[J]. Bioresource Technology, 160: 191-202.
DOI URL |
| [22] |
NGUYEN V H, THI L A P, LE Q V, et al., 2020. Tailored photocatalysts and revealed reaction pathways for photodegradation of polycyclic aromatic hydrocarbons (PAHs) in water, soil and other sources[J]. Chemosphere, 260: 127529.
DOI URL |
| [23] | OZAKI N, NAKAZATO A, NAKASHIMA K, et al., 2017. Loading and removal of PAHs, fragrance compounds, triclosan and toxicity by composting process from sewage sludge[J]. Science of the Total Environment, 605: 860-866. |
| [24] |
POTIN O, VEIGNIE E, RAFIN C, 2004. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Cladosporium sphaerospermum isolated from an aged PAH contaminated soil[J]. FEMS Microbiology Ecology, 51(1): 71-78.
DOI URL |
| [25] |
RAO M A, DI R S G, SCELZA R, et al., 2017. Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol[J]. Chemosphere, 186: 193-201.
DOI URL |
| [26] |
SCHWANNINGER M, RODRIGUES J C, PEREIRA H, et al., 2004. Effects of short-time vibratory ball milling on the shape of FTIR spectra of wood and cellulose[J]. Vibrational Spectroscopy, 36(1): 23-40.
DOI URL |
| [27] |
TANG J C, LV H H, GONG Y Y, et al., 2015. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal[J]. Bioresource Technology, 196: 355-363.
DOI URL |
| [28] |
TSAI W T, LIU S C, CHEN H R, et al., 2012. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment[J]. Chemosphere, 89: 198-203.
DOI URL |
| [29] |
YANG X, KWON E E, DOU X M, et al., 2018. Fabrication of spherical biochar by a two-step thermal process from waste potato peel[J]. Science of Total Environment, 626: 478-485.
DOI URL |
| [30] |
YUAN H R, LU T, ZHAO D D, et al., 2013. Influence of temperature on product distribution and biochar properties by municipal sludge pyrolysis[J]. Journal of Material Cycles and Waste Management, 15: 357-361.
DOI URL |
| [31] |
ZHANG J M, LIU G J, WANG R J, et al., 2017. Polycyclic aromatic hydrocarbons in the water-SPM-sediment system from the middle reaches of Huai River, China: distribution, partitioning, origin tracing and ecological risk assessment[J]. Environmental Pollution, 230: 61-71.
DOI URL |
| [32] |
ZHANG J, LIU J, LIU R L, 2015. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate[J]. Bioresource Technology, 176: 288-291.
DOI URL |
| [33] | ZHANG L, TONG L, ZHU P G, et al., 2018. Adsorption of chlortetracycline onto biochar derived from corn cob and sugarcane bagasse[J]. Water Science & Technology, 78(5-6): 1336-1347. |
| [34] |
ZHANG P, O’CONNOR D, WANG Y N, et al., 2020. A green biochar/iron oxide composite for methylene blue removal[J]. Journal of Hazardous Materials, 384: 121286.
DOI URL |
| [35] | 曹云者, 柳晓娟, 谢云峰, 等, 2012. 我国主要地区表层土壤中多环芳烃组成及含量特征分析[J]. 环境科学学报, 32(1): 197-203. |
| CAO Y Z, LIU X J, XIE Y F, et al., 2012. Patterns of PAHs concentrations and components in surface soils of main areas in China[J]. Acta Scientiae Circumstantiae, 32(1): 197-203. | |
| [36] | 陈再明, 陈宝梁, 周丹丹, 2013. 水稻秸秆生物碳的结构特征及其对有机污染物的吸附性能[J]. 环境科学学报, 33(1): 9-19. |
| CHEN Z M, CHEN B L, ZHOU D D, 2013. Composition and sorption properties of rice-straw derived biochars[J]. Acta Scientiae Circumstantiae, 33(1): 9-19. | |
| [37] | 戴中民, 2017. 生物炭对酸化土壤的改良效应与生物化学机理研究[D]. 杭州: 浙江大学. |
| DAI Z M, 2017. The effects of biochar on acid soil improvement and the related biochemical mechanisms[D]. Hangzhou: Zhejiang Uniwersity. | |
| [38] | 丁思惠, 方升佐, 田野, 等, 2020. 不同热解温度下杨树各组分生物炭的理化特性分析与评价[J]. 南京林业大学学报(自然科学版), 44(6): 193-200. |
| DING S H, FANG S Z, TIAN Y, et al., 2020. Analysis and evaluation on physicochemical properties of poplar biochar at different pyrolysis temperatures[J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 44(6): 193-200. | |
| [39] | 韩林, 2017. 生物炭和改性生物炭对有机污染物的吸附-转化性能及作用机理[D]. 杭州: 浙江大学. |
| HAN L, 2017. Adsorption-transformation performance and mechanism of organic polutants on biochar and modified biochar[D]. Hangzhou: Zhejiang Uniwersity. | |
| [40] | 杭嘉祥, 李法云, 梁晶, 等, 2020. 镁改性芦苇生物炭对水环境中磷酸盐的吸附特性[J]. 生态环境学报, 29(6): 1235-1244. |
| HANG J X, LI F Y, LIANG J, et al., 2020. The characteristics of phosphate adsorption in water environment by magnesium modified biochar from wetland reed[J]. Ecology and Environmental Sciences, 29(6): 1235-1244. | |
| [41] | 黄华, 王雅雄, 唐景春, 等, 2014. 不同烧制温度下玉米秸秆生物炭的性质及对萘的吸附性能[J]. 环境科学, 35(5): 1884-1890. |
| HUANG H, WANG Y X, TANG J C, et al., 2014. Properties of maize stalk biochar produced under different pyrolysis temperatures and its sorption capability to naphthalene[J]. Environmental Science, 35(5): 1884-1890. | |
| [42] | 李洋, 宋洋, 王芳, 等, 2015. 小麦秸秆生物炭对高氯代苯的吸附过程与机制研究[J]. 土壤学报, 52(5): 1096-1105. |
| LI Y, SONG Y, WANG F, et al., 2015. Effect of wheat straw biochar on high chlorinated benzene sorption process and mechanism[J]. Acta Pedologica Sinica, 52(5): 1096-1105. | |
| [43] | 蒲生彦, 上官李想, 刘世宾, 等, 2019. 生物炭及其复合材料在土壤污染修复中的应用研究进展[J]. 生态环境学报, 28(3): 629-635. |
| PU S Y, SAHNGGUAN L X, LIU S B, et al., 2019. A review of the application of biochar and its composites in soil remediation[J]. Ecology and Environmental Sciences, 28(3): 629-635. | |
| [44] | 乔印虎, 张春燕, 何春霞, 等, 2019. 预处理与低温热解对向日葵秸秆炭N2的吸附性能研究[J]. 太阳能学报, 40(8): 2128-2134. |
| QIAO Y H, ZHANG C Y, HE C X, et al., 2019. Effect of different pre-treatments and low-temperature pyrolysis on sunflower straw biochar for N2 adsorption[J]. Acta Energiae Solaris Sinica, 40(8): 2128-2134. | |
| [45] | 王蒙, 2018. 碱改性生物炭的制备及其作为替代硅源的潜能研究[D]. 杨凌: 西北农林科技大学. |
| WANG M, 2018. Alkali-enhanced biochar production and its potential as an alternative silicon source[D]. Yangling: Northwest A & F University. | |
| [46] | 王毅斌, 王学斌, 谭厚章, 等, 2015. 生物质燃烧过程中碱金属的结晶行为[J]. 燃烧科学与技术, 21(5): 435-439. |
| WANG Y B, WANG X B, TAN H Z, et al., 2015. Condensation behaviors of alkali salt vapors in biomass combustion[J]. Journal of Combustion Science and Technology, 21(5): 435-439. | |
| [47] | 韦思业, 2017. 不同生物质原料和制备温度对生物炭物理化学特征的影响[D]. 广州: 中国科学院大学(中国科学院广州地球化学研究所). |
| WEI S Y, 2017. Influence of biomass feedstocks and pyrolysis temperatures on physical and chemical properties of biochar[D]. Guangzhou: University of Chinese Academy of Sciences (Guangzhou Institute of Geochemistry, | |
| [48] | 吴晴雯, 孟梁, 张志豪, 等, 2016. 芦苇秸秆生物炭对水中菲和1, 1-二氯乙烯的吸附特性[J]. 环境科学, 37(2): 680-688. |
| WU Q W, MENG L, ZHANG Z H, et al., 2016. Sorption characteristics of phenanthrene and 1, 1-dichloroethene onto reed straw biochar in aquatic solutions[J]. Environmental Science, 37(2): 680-688. |
| [1] | 赵维彬, 唐丽, 王松, 刘玲玲, 王树凤, 肖江, 陈光才. 两种生物炭对滨海盐碱土的改良效果[J]. 生态环境学报, 2023, 32(4): 678-686. |
| [2] | 付蓉, 武新梅, 陈斌. 城市地表温度空间分异及驱动因子差异性分析——以合肥市为例[J]. 生态环境学报, 2023, 32(1): 110-122. |
| [3] | 蒋恬田, 杨纯, 廖炜, 胡力, 刘欢瑶, 任勃, 李小马. 城市绿地景观格局影响地表温度的通径分析——以长沙市为例[J]. 生态环境学报, 2023, 32(1): 18-25. |
| [4] | 游宏建, 张文文, 兰正芳, 马兰, 张宝娣, 穆晓坤, 李文慧, 曹云娥. 蚯蚓原位堆肥与生物炭对黄瓜根结线虫及根际微生物的影响[J]. 生态环境学报, 2023, 32(1): 99-109. |
| [5] | 李晓晖, 艾仙斌, 李亮, 王玺洋, 辛在军, 孙小艳. 新型改性稻壳生物炭材料对镉污染土壤钝化效果的研究[J]. 生态环境学报, 2022, 31(9): 1901-1908. |
| [6] | 阮惠华, 许剑辉, 张菲菲. 2001—2020年粤港澳大湾区植被和地表温度时空变化研究[J]. 生态环境学报, 2022, 31(8): 1510-1520. |
| [7] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| [8] | 房献宝, 张智钧, 赖阳晴, 叶脉, 刁增辉. 新型污泥生物炭对土壤重金属Cr和Cd的修复研究[J]. 生态环境学报, 2022, 31(8): 1647-1656. |
| [9] | 崔乔, 李宗省, 张百娟, 赵越, 南富森. 冻融作用对土壤可溶性碳氮和微生物量碳氮含量影响的荟萃分析[J]. 生态环境学报, 2022, 31(8): 1700-1712. |
| [10] | 钱莲文, 余甜甜, 梁旭军, 王义祥, 陈永山. 茶园土壤酸化改良中生物炭应用5 a后的稳定性研究[J]. 生态环境学报, 2022, 31(7): 1442-1447. |
| [11] | 雷俊, 张健, 赵福年, 齐月, 张秀云, 李强, 尚军林. 春小麦开花期光合参数对土壤水分和温度变化的响应[J]. 生态环境学报, 2022, 31(6): 1151-1159. |
| [12] | 张慧琦, 李子忠, 秦艳. 玉米秸秆生物炭用量对砂土孔隙和持水性的影响[J]. 生态环境学报, 2022, 31(6): 1272-1277. |
| [13] | 陈丽娟, 周文君, 易艳芸, 宋清海, 张一平, 梁乃申, 鲁志云, 温韩东, MOHD Zeeshan, 沙丽清. 云南哀牢山亚热带常绿阔叶林土壤CH4通量特征[J]. 生态环境学报, 2022, 31(5): 949-960. |
| [14] | 李喆, 陈圣宾, 陈芝阳. 地表温度与土地利用类型间的空间尺度依赖性——以成都为例[J]. 生态环境学报, 2022, 31(5): 999-1007. |
| [15] | 邓晓, 武春媛, 杨桂生, 李怡, 李勤奋. 椰壳生物炭对海南滨海土壤的改良效果[J]. 生态环境学报, 2022, 31(4): 723-731. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
