Ecology and Environment ›› 2022, Vol. 31 ›› Issue (1): 151-159.DOI: 10.16258/j.cnki.1674-5906.2022.01.017
• Research Articles • Previous Articles Next Articles
CHEN Wenjie1,2( ), LI Hui3, HE Bin2, TAO Liang2,4,*(
), LI Hui3, HE Bin2, TAO Liang2,4,*( )
)
Received:2021-10-25
Online:2022-01-18
Published:2022-03-10
Contact:
TAO Liang
通讯作者:
陶亮
作者简介:陈文洁(1998年生),女,硕士研究生,主要从事土壤界面化学过程研究。E-mail: 1614230745@qq.com
基金资助:CLC Number:
CHEN Wenjie, LI Hui, HE Bin, TAO Liang. Influence of Co-existing Anions and Cations on Phosphate Sequestration onto Goethite[J]. Ecology and Environment, 2022, 31(1): 151-159.
陈文洁, 李慧, 贺斌, 陶亮. 共存阴阳离子对针铁矿表面磷固存机制的影响研究[J]. 生态环境学报, 2022, 31(1): 151-159.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.jeesci.com/EN/10.16258/j.cnki.1674-5906.2022.01.017
| 矿物 Minerals | Qe/(μmol∙g-1) | SSA/(m2∙g-1) | Qe’/(μmol∙m-2) |
|---|---|---|---|
| 针铁矿 Goethite | 232.04 | 81.15 | 2.86 |
| 赤铁矿 Hematite | 18.71 | 29.19 | 0.64 |
| 高岭石 Kaolinite | 2.78 | 15.70 | 0.18 |
Table 1 The normalized adsorption efficiency of P (V) onto different mineral surfaces (pH=6.0)
| 矿物 Minerals | Qe/(μmol∙g-1) | SSA/(m2∙g-1) | Qe’/(μmol∙m-2) |
|---|---|---|---|
| 针铁矿 Goethite | 232.04 | 81.15 | 2.86 |
| 赤铁矿 Hematite | 18.71 | 29.19 | 0.64 |
| 高岭石 Kaolinite | 2.78 | 15.70 | 0.18 |
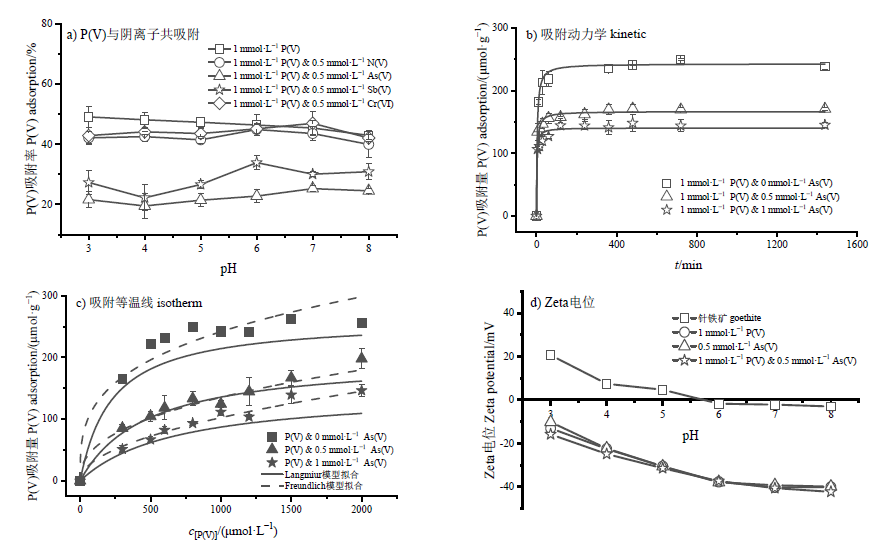
Figure 3 Effects of anions on P(V) adsorption onto goethite (a), kinetics of P(V) adsorption (b), isotherms of P(V) adsorption when P(V) co-adsorbed with As(V) onto goethite (c), and Zeta potential values when P(V) co-adsorbed with As(V) onto goethite surface (d)
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ (min-1) | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 88.89 | 0.4954 | 0.835 | 166.67 | 0.0026 | 0.999 | |
| P(V)3 | 72.59 | 0.4290 | 0.701 | 140.65 | 0.0038 | 0.996 | |
Table 2 Fitting parameter of P(V) adsorption kinetic when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ (min-1) | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 88.89 | 0.4954 | 0.835 | 166.67 | 0.0026 | 0.999 | |
| P(V)3 | 72.59 | 0.4290 | 0.701 | 140.65 | 0.0038 | 0.996 | |
| 吸附质 Adsorbate | Langmuir模型 Langmuir model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| Qm/ (μmol∙g-1) | KL/ (L∙μmol-1) | R2 | n | KF/(μmol∙g-1)∙ (μmol∙L-1)n | R2 | ||
| P(V)1 | 262.10 | 0.0045 | 0.988 | 3.29 | 29.82 | 0.900 | |
| P(V)2 | 197.97 | 0.0021 | 0.922 | 2.44 | 7.98 | 0.917 | |
| P(V)3 | 146.47 | 0.0015 | 0.895 | 1.99 | 3.19 | 0.904 | |
Table 3 The fitting parameter of P(V) adsorption isotherm when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | Langmuir模型 Langmuir model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| Qm/ (μmol∙g-1) | KL/ (L∙μmol-1) | R2 | n | KF/(μmol∙g-1)∙ (μmol∙L-1)n | R2 | ||
| P(V)1 | 262.10 | 0.0045 | 0.988 | 3.29 | 29.82 | 0.900 | |
| P(V)2 | 197.97 | 0.0021 | 0.922 | 2.44 | 7.98 | 0.917 | |
| P(V)3 | 146.47 | 0.0015 | 0.895 | 1.99 | 3.19 | 0.904 | |
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ min-1 | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 130.69 | 0.3042 | 0.775 | 240.96 | 0.0010 | 0.999 | |
| P(V)3 | 402.49 | 0.5954 | 0.899 | 251.89 | 0.0041 | 0.999 | |
| P(V)4 | 174.82 | 0.6929 | 0.406 | 271.74 | 0.0003 | 0.999 | |
Table 4 The fitting parameter of P(V) adsorption kinetic when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ min-1 | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 130.69 | 0.3042 | 0.775 | 240.96 | 0.0010 | 0.999 | |
| P(V)3 | 402.49 | 0.5954 | 0.899 | 251.89 | 0.0041 | 0.999 | |
| P(V)4 | 174.82 | 0.6929 | 0.406 | 271.74 | 0.0003 | 0.999 | |
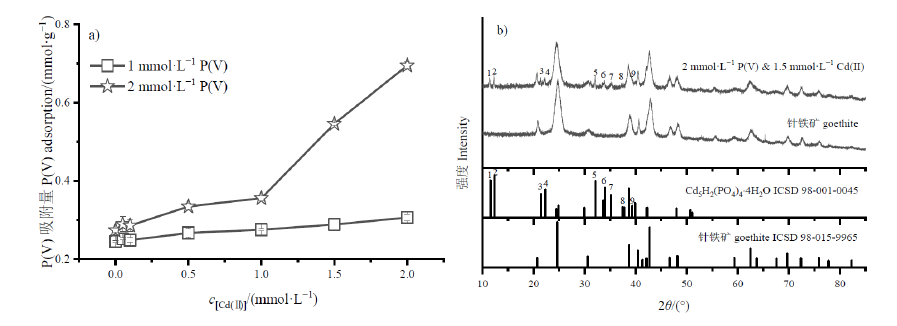
Figure 5 Effects of different Cd(II) concentrations on P(V) adsorption (a); XRD patterns (b) of 2 mmol∙L-1 P(V) and 1.5 mmol∙L-1 Cd(II) adsorbed onto goethite
| [1] |
ANTELO J, AVENA M, FIOL S, et al., 2005. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface[J]. Journal of Colloid and Interface Science, 285(2): 476-486.
DOI URL |
| [2] |
ATOUEI M T, RAHNEMAIE R, KALANPA E G, et al., 2016. Competitive adsorption of magnesium and calcium with phosphate at the goethite water interface: Kinetics, equilibrium and CD-MUSIC modeling[J]. Chemical Geology, 437(10): 19-29.
DOI URL |
| [3] | CORNELL R M, SCHWERTMANN U, 2003. The iron oxides: structure, properties, reactions, occurrences and uses[M]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA: 14-29, 531-535. |
| [4] | COSTA E T S, GUILHERME L R G, LOPES G, et al., 2012. Competitive sorption of arsenate and phosphate on aluminum mining by-product[J]. Water, Air, & Soil Pollution, 223(8): 5433-5444. |
| [5] |
ELZINGA E J, KRETZSCHMAR R, 2013 In situ ATR-FTIR spectroscopic analysis of the co-adsorption of orthophosphate and Cd(II) onto hematite[J]. Geochimica et Cosmochimica Acta, 117(9): 53-64.
DOI URL |
| [6] |
ESSINGTON M E, VERGEER K A, 2015. Adsorption of antimonate, phosphate, and sulfate by manganese dioxide: competitive effects and surface complexation modeling[J]. Soil Science Society of America Journal, 79(3): 803-814.
DOI URL |
| [7] | FLATEN D, SHARPLEY A, JARVIE H, et al., 2019. Reducing unintended consequences of agricultural phosphorus[J]. Better Crops Plant Food, 103(1): 33-35. |
| [8] |
HEALTHMAN G C, SHARPLEY A N, SMITH S J, et al., 1994. Land application of poultry litter application and water quality in Oklahoma, U.S.A.[J]. Fertilizer Research, 40(3): 165-173.
DOI URL |
| [9] |
JARVIE H P, SHARPLEY A N, FLATEN D, et al., 2019. Phosphorus mirabilis: Illuminating the past and future of phosphorus stewardship[J]. Journal of Environmental Quality, 48(5): 1127-1132.
DOI URL |
| [10] |
JARVIE H P, SHARPLEY A N, WITHERS P J A, et al., 2013. Phosphorus mitigation to control river eutrophication: Murky waters, inconvenient truths, and “postnormal” science[J]. Journal of Environmental Quality, 42(2): 295-304.
DOI URL |
| [11] |
LI L, STANFORTH R, 2000. Distinguishing adsorption and surface precipitation of phosphate on goethite (α-FeOOH)[J]. Journal of Colloid and Interface Science, 230(1): 12-21.
DOI URL |
| [12] |
LIU J, ZHU R L, XU T Y, et al., 2016 Co-adsorption of phosphate and zinc (II) on the surface of ferrihydrite[J]. Chemosphere, 144(2): 1148-1155.
DOI URL |
| [13] |
LIU Y T, HESTERBERG D, 2011. Phosphate bonding on non-crystalline Al/Fe-hydroxide coprecipitates[J]. Environmental Science & Technology, 45(15): 6283-6289.
DOI URL |
| [14] |
MANNING B A, GOLDBERG S, 1996. Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals[J]. Soil Science Society of America Journal, 60(1): 121-131.
DOI URL |
| [15] |
MEKONNEN M M, HOEKSTRA A Y, 2018. Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: A high-resolution global study[J]. Water Resources Research, 54(1): 345-358.
DOI URL |
| [16] |
PINTOR A M A, BRANDÃO C C, BOAVENTURA R A R, et al., 2021. Multicomponent adsorption of pentavalent As, Sb and P onto iron-coated cork granulates[J]. Journal of Hazardous Materials, DOI: 10.1016/j.jhazmat.2020.124339.
DOI |
| [17] |
QIU J, 2010. Phosphate fertilizer warning for China[J]. Nature, DOI: 10.1038/news.2010.498.
DOI |
| [18] |
RUSSELL J D, PARFITT R L, FRASER A R, et al., 1974. Surface structures of gibbsite goethite and phosphated goethite[J]. Nature, 248(5445): 220-221.
DOI URL |
| [19] |
SHARPLEY A, JARVIE H, FLATEN D, et al., 2018. Celebrating the 350th anniversary of phosphorus discovery: A conundrum of deficiency and excess[J]. Journal of Environmental Quality, 47(4): 774-777.
DOI URL |
| [20] |
SMITH D R, MACRAE M L, KLEINMAN P J A, et al., 2019. The latitudes, attitudes, and platitudes of watershed phosphorus management in North America[J]. Journal of Environmental Quality, 48(5): 1176-1190.
DOI URL |
| [21] | STOCKDALE E A, SHEPHERD M A, FORTUNE S, et al., 2002. Soil fertility in organic farming systems-fundamentally different?[J]. Soil Use and Management, 18(1): 301-308. |
| [22] |
TAO L, LI F B, WANG Y K, et al., 2010 Reductive activity of adsorbed Fe (II) on iron (oxyhydr) oxides for 2-nitrophenol transformation[J]. Clays and Clay Minerals, 58(5): 682-690.
DOI URL |
| [23] |
TAO L, WEN X C, LI H, et al., 2021. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment[J]. Applied Clay Science, 204: 106021.
DOI URL |
| [24] |
TEJEDOR-TEJEDOR M I, ANDERSON M A, 1990. The protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobility[J]. Langmuir, 6(3): 602-611.
DOI URL |
| [25] |
VIOLANTE A, PIGNA M, 2002. Competitive sorption of arsenate and phosphate on different clay minerals and soils[J]. Soil Science Society of America Journal, 66(6): 1788-1796.
DOI URL |
| [26] |
WEI S Y, TAN W F, LIU F, et al., 2014. Surface properties and phosphate adsorption of binary systems containing goethite and kaolinite[J]. Geoderma, 213(1): 478-484.
DOI URL |
| [27] |
YU N Y, WU K, TAO L, 2021. Synchronous reduction-fixation of reducible heavy metals from aqueous solutions: Application of novel mesoporous MFT/SBA-15 composite materials[J]. Chemosphere, 276: 130112.
DOI URL |
| [28] |
ZHANG W F, MA W Q, JI Y X, et al., 2008. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China[J]. Nutrient Cycling in Agroecosystems, 80(2): 131-144.
DOI URL |
| [29] |
ZHANG W F, TANG X M, FENG X H, et al., 2019. Management strategies to optimize soil phosphorus utilization and alleviate environmental risk in China[J]. Journal of Environmental Quality, 48(5): 1167-1175.
DOI URL |
| [30] |
ZHU M X, DING K Y, JIANG X, et al., 2007. Investigation on co-sorption and desorption of fluoride and phosphate in a red soil of China[J]. Water, Air, and Soil Pollution, 183(1): 455-465.
DOI URL |
| [31] | 黄国勤, 王兴祥, 钱海燕, 等, 2004. 施用化肥对农业生态环境的负面影响及对策[J]. 生态环境, 13(4): 656-660. |
| HUANG G Q, WANG X X, QIAN H Y, et al., 2004. Negative impact of inorganic fertilizes application on agricultural environment and its countermeasures[J]. Ecology and Environment, 13(4): 656-660. | |
| [32] |
黄敏雪, 管玉峰, 苏子贤, 等, 2022. 砷镉在不同矿物界面的相互作用过程[J/OL]. 土壤学报, DOI: 10.11766/trxb202101140027.
DOI |
|
HUANG M X, GUAN Y F, SU Z X, et al., 2022. Interfacial reactions between As(V) and Cd(II) co-adsorption onto various mineral surfaces[J/OL]. Acta Pedologica Sinica, DOI: 10.11766/trxb202101140027.
DOI |
|
| [33] | 吕贻忠, 李保国, 2006. 土壤学[M]. 北京: 中国农业出版社: 244-249. |
| LÜ Y Z, LI B G, 2006. Agrology[M]. Beijing: China Agriculture Press: 244-249. | |
| [34] | 全为民, 严力蛟, 2002. 农业面源污染对水体富营养化的影响及其防治措施[J]. 生态学报, 22(3): 291-299. |
| QUAN W M, YAN L J, 2002. Effects of agricultural non-point source pollution on eutrophication of water body and its control measure[J]. Acta Ecologica Sinica, 22(3): 291-299. | |
| [35] | 石华, 1989. 红壤研究四十春为庆祝建国40周年而作[J]. 土壤 (4): 174-179. |
| SHI H, 1989. Red soil research forty spring to celebrate the 40th anniversary of the founding of the people's Republic of China[J]. Soil (4): 174-179. | |
| [36] | 司友斌, 王慎强, 陈怀满, 2000. 农田氮、磷的流失与水体富营养化[J]. 土壤, 32(4): 188-193. |
| SI Y B, WANG S Q, CHEN H M, 2000. Loss of nitrogen and phosphorus in farmland and water eutrophication[J]. Soil, 32(4): 188-193. | |
| [37] | 王小玲, 马杰, 顾明华, 等, 2015. 砷和磷在不同污染类型土壤中的竞争吸附动力学[J]. 生态环境学报, 24(4): 694-699. |
| WANG X L, MA J, GU M H, et al., 2015. Competitive adsorption kinetics of arsenic and phosphorus in different kinds of contaminated soils[J]. Ecology and Environmental Sciences, 24(4): 694-699. | |
| [38] | 严玉鹏, 王小明, 熊娟, 等, 2020. 基于不同分析方法研究磷酸根在矿物表面吸附机制的进展[J]. 土壤学报, 57(1): 22-35. |
| YAN Y P, WANG X M, XIONG J, et al., 2020. Progresses in studies on sorption mechanisms of phosphate on minerals using multiple analytic approaches[J]. Acta Pedologica Sinica, 57(1): 22-35. |
| [1] | DU Dandan, GAO Ruizhong, FANG Lijing, XIE Longmei. Spatial Variation of Soil Heavy Metals and Their Responses to Physicochemical Factors of Salt Lake Basin in Arid Area [J]. Ecology and Environment, 2023, 32(6): 1123-1132. |
| [2] | FENG Shuna, LÜ Jialong, HE Hailong. Effect of KI Leaching on the Hg (Ⅱ) Removal of Loess Soil and the Physicochemical Properties of the Soil [J]. Ecology and Environment, 2023, 32(4): 776-783. |
| [3] | CHEN Minyi, ZHU Hanghai, SHE Weiduo, YIN Guangcai, HUANG Zuzhao, YANG Qiaoling. Health Risk Assessment and Source Apportionment of Soil Heavy Metals at A Legacy Shipyard Site in Pearl River Delta [J]. Ecology and Environment, 2023, 32(4): 794-804. |
| [4] | XIAO Jieyun, ZHOU Wei, SHI Peiqi. Hyperspectral Inversion of Soil Heavy Metals [J]. Ecology and Environment, 2023, 32(1): 175-182. |
| [5] | HAUNG Hong, ZHENG Xinyun, LI Yingdong, ZHAO Xu, YU Jinchen, WANG Zhenhua. A study on Enrichment of Heavy Metals in Sebastiscus marmoratus at Different Ages in Dachen Islands Sea Area [J]. Ecology and Environment, 2022, 31(9): 1885-1891. |
| [6] | MA Chuang, WANG Yuyang, ZHOU Tong, WU Longhua. Enrichment Characteristics and Desorption Behavior of Cadmium and Zinc in Particulate Organic Matter of Polluted Soil [J]. Ecology and Environment, 2022, 31(9): 1892-1900. |
| [7] | TAO Ling, HUANG Lei, ZHOU Yilei, LI Zhongxing, REN Jun. Influences of Biochar Prepared by Co-pyrolysis with Sludge and Attapulgite on Bioavailability and Environmental Risk of Heavy Metals in Mining Soil [J]. Ecology and Environment, 2022, 31(8): 1637-1646. |
| [8] | LI Ying, ZHANG Zhou, YANG Gaoming, ZU Yanqun, LI Bo, CHEN Jianjun. The Relationship between the Radial Oxygen Loss and the Iron Plaque on Root Surfaces to Wetland Plants Absorb Heavy Metals [J]. Ecology and Environment, 2022, 31(8): 1657-1666. |
| [9] | LUO Songying, LI Qiuxia, QIU Jinkun, DENG Suyan, LI Yifeng, CHEN Bishan. Speciation Characteristics, Migration and Transformation of Heavy Metals in Mangrove Soil-plant System in Nansan Island [J]. Ecology and Environment, 2022, 31(7): 1409-1416. |
| [10] | DONG Leheng, WANG Xugang, CHEN Manjia, WANG Zihao, SUN Lirong, SHI Zhaoyong, Wu Qiqi. Interaction of Iron Redox and Cu Activities in Calcareous Paddy Soil under Light and Dark Condition [J]. Ecology and Environment, 2022, 31(7): 1448-1455. |
| [11] | PENG Hongli, TAN Haixia, WANG Ying, WEI Jianmei, FENG Yang. The Discrepancy of Heavy Metals Morphological Distribution in Soil and Its Associated Ecological Risk Evaluation under Different Planting Patterns [J]. Ecology and Environment, 2022, 31(6): 1235-1243. |
| [12] | HUANG Min, ZHAO Xiaofeng, LIANG Rongxiang, WANG Pengzhong, DAI Anran, HE Xiaoman. Comparison of Three Chelating Agents to Remove the Cd and Cu in Contaminated Soil [J]. Ecology and Environment, 2022, 31(6): 1244-1252. |
| [13] | ZHU Li'an, ZHANG Huihua, CHENG Jiong, LI Ting, LIN ZI, LI Junjie. Potential Ecological Risk Pattern Analysis of Heavy Metals in Soil of Forestry Land in The Pearl River Delta [J]. Ecology and Environment, 2022, 31(6): 1253-1262. |
| [14] | SHI Jianfei, JIN Zhengzhong, ZHOU Zhibin, WANG Xin. Evaluation of Heavy Metal Pollution in the Soil Around A Typical Tailing Reservoir in Irtysh River Basin [J]. Ecology and Environment, 2022, 31(5): 1015-1023. |
| [15] | QIAN Xueshi, LI Yong, QIAN Zhuangzhuang, GE Xiaomin, TANG Luozhong. Changes of Cadmium, Lead and Arsenic Contents during Precipitation in the Secondary Broad-leaved Forest in the Eastern Area of North Subtropics, China [J]. Ecology and Environment, 2022, 31(5): 979-989. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2021 Editorial Office of ACTA PETROLEI SINICA
Address:No. 6 Liupukang Street, Xicheng District, Beijing, P.R.China, 510650
Tel: 86-010-62067128, 86-010-62067137, 86-010-62067139
Fax: 86-10-62067130
Email: syxb@cnpc.com.cn
Support byBeijing Magtech Co.ltd, E-mail:support@magtech.com.cn
