生态环境学报 ›› 2022, Vol. 31 ›› Issue (9): 1876-1884.DOI: 10.16258/j.cnki.1674-5906.2022.09.018
王钊1,2,3( ), 张曼胤1,2,3,*(
), 张曼胤1,2,3,*( ), 胡宇坤1,2,3, 刘魏魏1,2,3, 张苗苗1,2,3
), 胡宇坤1,2,3, 刘魏魏1,2,3, 张苗苗1,2,3
收稿日期:2022-04-06
出版日期:2022-09-18
发布日期:2022-11-07
通讯作者:
*张曼胤(1979年生),男,研究员,博士,硕士研究生导师,研究方向为湿地生态功能作用机理、湿地监测与评价、湿地保护规划、湿地恢复设计等。E-mail: cneco@126.com作者简介:王钊(1996年生),男,硕士研究生,主要研究方向为湿地生态学。E-mail: wangz435@nenu.edu.cn
基金资助:
WANG Zhao1,2,3( ), ZHANG Manyin1,2,3,*(
), ZHANG Manyin1,2,3,*( ), HU Yukun1,2,3, LIU Weiwei1,2,3, ZHANG Miaomiao1,2,3
), HU Yukun1,2,3, LIU Weiwei1,2,3, ZHANG Miaomiao1,2,3
Received:2022-04-06
Online:2022-09-18
Published:2022-11-07
摘要:
盐度是滨海湿地最重要的环境特征之一,然而滨海湿地盐度的变化如何影响汞甲基化过程目前仍缺乏相关研究。基于室内培养的方法,研究了厌氧条件下江苏盐城滨海湿地沉积物甲基汞质量分数和硫酸盐还原菌绝对含量随盐度梯度(0、0.6%、1.2%、1.8%、2.4%和3.0%)的变化特征。结果表明,海盐处理下,随着盐度的升高,甲基汞质量分数总体呈现先增加后减少的趋势,1.2%盐度下,沉积物汞甲基化程度最高;随着培养时间(1-29 d)的增长,甲基汞质量分数总体也呈现出先增加后减少的趋势,培养8 d时,沉积物汞甲基化程度最高。海盐处理下,随着盐度的升高,硫酸盐还原菌的绝对含量总体增加,表明海水盐度未对硫酸盐还原菌生长产生盐胁迫;随着培养时间(1-29 d)的增加,硫酸盐还原菌的绝对含量表现为先增加后减少的趋势,当培养时间超过15 d时,硫酸盐还原菌的生长会受到抑制。该实验条件下,硫酸盐还原菌绝对含量与甲基汞质量分数间无显著线性相关,可溶性有机碳对甲基汞质量分数随时间的变化具有较高的解释度。该研究表明滨海湿地盐度的时空变化对汞甲基化过程有较重要的影响。研究结果可为预测盐度变化所带来的甲基汞生态风险提供了依据。
中图分类号:
王钊, 张曼胤, 胡宇坤, 刘魏魏, 张苗苗. 盐度对典型滨海湿地沉积物汞甲基化的影响[J]. 生态环境学报, 2022, 31(9): 1876-1884.
WANG Zhao, ZHANG Manyin, HU Yukun, LIU Weiwei, ZHANG Miaomiao. Effect of Salinity on Mercury Methylation in Sediments of A Typical Coastal Wetland[J]. Ecology and Environment, 2022, 31(9): 1876-1884.
| 样地 Sample plots | 样本量 Sample size | 经纬度 Longitude and latitude | 主要植被类型 Main vegetation type |
|---|---|---|---|
| 新洋港北 North of Xinyang Port | 5 | 120.5660°-120.5666°E, 33.6356°-33.6362°N | 芦苇 Phragmites australis、盐度碱蓬 Suaeda salsa |
| 核心保护区 Core Reserve | 10 | 120.5537°-120.6141°E, 33.5778°-33.6090°N | 芦苇 Phragmites australis、互花米草 Spartina alterniflora、盐地碱蓬 Suaeda salsa |
| 大丰麋鹿区 Dafeng Elk Natural Reserve | 5 | 120.8491°-120.8622°E, 33.0529°-33.0550°N | 互花米草 Spartina alterniflora、白茅 Imperata cylindrica |
表1 野外样品采集情况
Table 1 Description of sample collection in the field
| 样地 Sample plots | 样本量 Sample size | 经纬度 Longitude and latitude | 主要植被类型 Main vegetation type |
|---|---|---|---|
| 新洋港北 North of Xinyang Port | 5 | 120.5660°-120.5666°E, 33.6356°-33.6362°N | 芦苇 Phragmites australis、盐度碱蓬 Suaeda salsa |
| 核心保护区 Core Reserve | 10 | 120.5537°-120.6141°E, 33.5778°-33.6090°N | 芦苇 Phragmites australis、互花米草 Spartina alterniflora、盐地碱蓬 Suaeda salsa |
| 大丰麋鹿区 Dafeng Elk Natural Reserve | 5 | 120.8491°-120.8622°E, 33.0529°-33.0550°N | 互花米草 Spartina alterniflora、白茅 Imperata cylindrica |
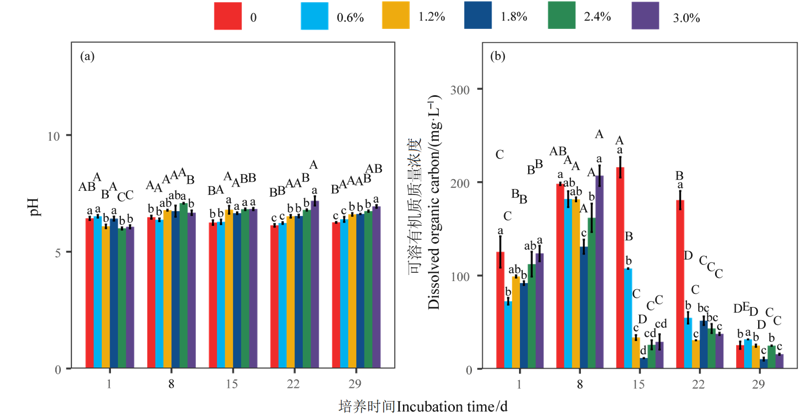
图2 不同盐度和时间下pH和DOC的差异 平均值±标准误,n=5。不同小写字母表示同时间不同盐度处理之间差异显著;不同大写字母表示同盐度不同时间处理之间差异显著。下同
Figure 2 Differences in pH and DOC among different salinity levels and incubation time Mean±SE, n=5. Different lowercase letters indicate that the same incubation time has significant differences among different salinity levels at 0.05 level; different capital letters indicate that the same salinity has significant differences among different incubation time at 0.05 level. The same below
| 时间 Time/d | 盐度 Salinity/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.6 | 1.2 | 1.8 | 2.4 | 3.0 | |||||||
| t | P | t | P | t | P | t | P | t | P | t | P | |
| 1 | 4.016 | 0.057ns | 21.496 | 0.002** | 18.796 | 0.003** | 2.219 | 0.157ns | 9.591 | 0.011* | 9.148 | 0.012* |
| 8 | 8.331 | 0.014* | 9.705 | 0.010* | 11.241 | 0.008** | 13.096 | 0.006** | 21.224 | 0.002** | 8.727 | 0.013* |
| 15 | 10.371 | 0.009** | 20.370 | 0.002** | 34.204 | <0.001*** | 5.398 | 0.033* | 29.100 | 0.001** | 5.408 | 0.033* |
| 22 | 5.620 | 0.030* | 3.402 | 0.080ns | 11.017 | 0.008** | 11.568 | 0.007** | 4.376 | 0.048* | 5.456 | 0.032* |
| 29 | 7.526 | 0.017* | 3.243ns | 0.083ns | -2.752 | 0.111ns | 2.349 | 0.143ns | 6.092 | 0.03* | 4.673 | 0.043* |
表2 模拟盐度下沉积物甲基汞质量分数与混合均质样本底值的差异
Table 2 Differences in methylmercury contents between the sediments under simulated salinity and the background level
| 时间 Time/d | 盐度 Salinity/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.6 | 1.2 | 1.8 | 2.4 | 3.0 | |||||||
| t | P | t | P | t | P | t | P | t | P | t | P | |
| 1 | 4.016 | 0.057ns | 21.496 | 0.002** | 18.796 | 0.003** | 2.219 | 0.157ns | 9.591 | 0.011* | 9.148 | 0.012* |
| 8 | 8.331 | 0.014* | 9.705 | 0.010* | 11.241 | 0.008** | 13.096 | 0.006** | 21.224 | 0.002** | 8.727 | 0.013* |
| 15 | 10.371 | 0.009** | 20.370 | 0.002** | 34.204 | <0.001*** | 5.398 | 0.033* | 29.100 | 0.001** | 5.408 | 0.033* |
| 22 | 5.620 | 0.030* | 3.402 | 0.080ns | 11.017 | 0.008** | 11.568 | 0.007** | 4.376 | 0.048* | 5.456 | 0.032* |
| 29 | 7.526 | 0.017* | 3.243ns | 0.083ns | -2.752 | 0.111ns | 2.349 | 0.143ns | 6.092 | 0.03* | 4.673 | 0.043* |
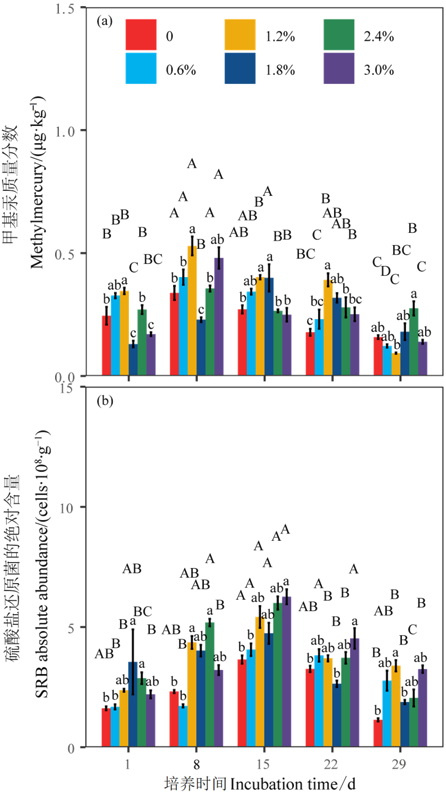
图3 不同盐度和时间下甲基汞质量分数与SRB绝对含量的差异
Figure 3 Differences in methylmercury content and absolute abundance of sulfate-reducing bacteria among different salinity levels and incubation time
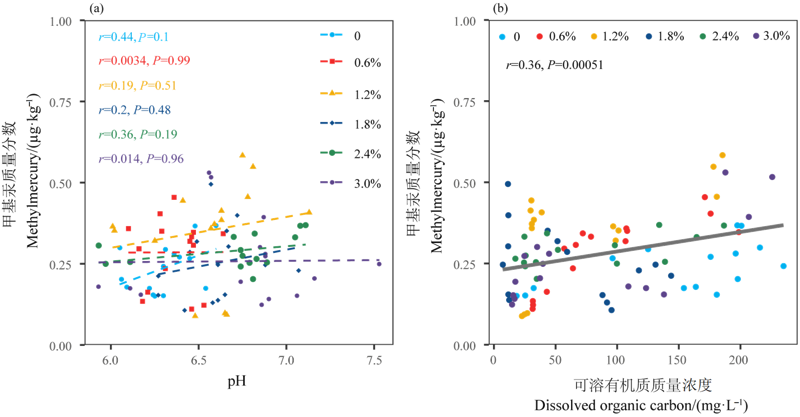
图4 pH和DOC与甲基汞质量分数之间的关系 n=3。r表示相关系数,P表示显著性检验大小
Figure 4 Relationships between pH, DOC and methylmercury content n=3. r represents the correlation coefficient; P represents the value of significant test
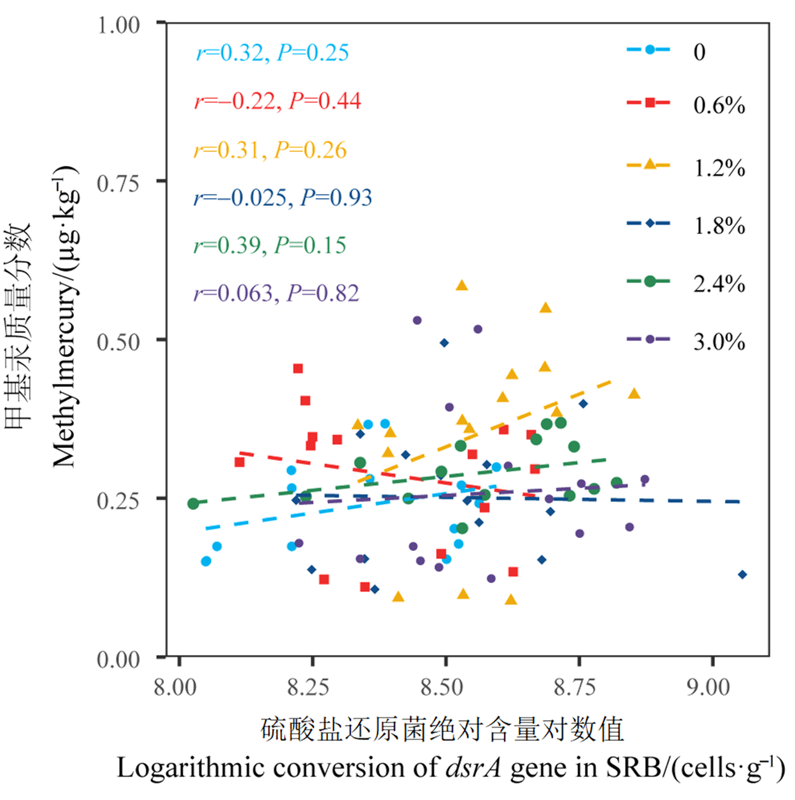
图5 SRB绝对含量与甲基汞质量分数之间的关系 n=3。r表示相关系数,P表示显著性检验大小,对数转换指取以10为底的对数
Figure 5 Relationship between absolute abundance of SRB and methylmercury content n=3. r represents the correlation coefficient; P represents the value of significant test. Logarithmic transform points to the base 10 logarithm
| [1] | AZAROFF A, GOÑI URRIZA M, GASSIE C, et al., 2020. Marine mercury-methylating microbial communities from coastal to Capbreton Canyon sediments (North Atlantic Ocean)[J]. Environmental Pollution, 262: 114333. |
| [2] |
BARKAY T, GILLMAN M, TURNER R R, 1997. Effects of dissolved organic carbon and salinity on bioavailability of mercury[J]. Applied and Environmental Microbiology, 63(11): 4267-4271.
DOI PMID |
| [3] |
BLUM J E, BARTHA R, 1980. Effect of salinity on methylation of mercury[J]. Bulletin of Environmental Contamination and Toxicology, 25(1): 404-408.
DOI URL |
| [4] | BOYD E S, YU R Q, BARKAY T, et al., 2017. Effect of salinity on mercury methylating benthic microbes and their activities in Great Salt Lake, Utah[J]. Science of The Total Environment, 581-582: 495-506. |
| [5] | BRAATEN H F V, DE WIT H A, FJELD E, et al., 2014. Environmental factors influencing mercury speciation in Subarctic and Boreal lakes[J]. Science of the Total Environment, 476-477: 336-345. |
| [6] | CASTRO H F, WILLIAMS N H, OGRAM A, 2000. Phylogeny of sulfate-reducing bacteria[J]. FEMS Microbiology Ecology, 31(1): 1-9. |
| [7] |
CHEN B, CHEN P, HE B, et al, 2015. Identification of mercury methylation product by tert-butyl compounds in aqueous solution under light irradiation[J]. Marine Pollution Bulletin, 98(1): 40-46.
DOI URL |
| [8] |
COMPEAU G, BARTHA R, 1983. Effects of sea salt anions on the formation and stability of methylmercury[J]. Bulletin of Environmental Contamination and Toxicology, 31(4): 486-493.
PMID |
| [9] |
COMPEAU G, BARTHA R, 1984. Methylation and demethylation of mercury under controlled redox, pH and salinity conditions[J]. Applied and Environmental Microbiology, 48(6): 1203-1207.
DOI PMID |
| [10] |
COMPEAU G C, BARTHA R, 1985. Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment[J]. Applied and Environmental Microbiology, 50(2): 498-502.
DOI PMID |
| [11] |
COMPEAU G C, BARTHA R, 1987. Effect of salinity on mercury-methylating activity of sulfate-reducing bacteria in estuarine sediments[J]. Applied and Environmental Microbiology, 53(2): 261-265.
DOI PMID |
| [12] |
DE OLIVEIRA D C M, CORREIA R R S, MARINHO C C, et al., 2015. Mercury methylation in sediments of a Brazilian mangrove under different vegetation covers and salinities[J]. Chemosphere, 127: 214-221.
DOI PMID |
| [13] |
FLEMING E J, MACK E E, GREEN P G, et al., 2006. Mercury methylation from unexpected sources: Molybdate-Inhibited freshwater sediments and an iron-reducing bacterium[J]. Applied and Environmental Microbiology, 72(1): 457-464.
PMID |
| [14] | GILMOUR C, BELL J T, SOREN A B, et al., 2018. Distribution and biogeochemical controls on net methylmercury production in Penobscot River marshes and sediment[J]. Science of The Total Environment, 640-641: 555-569. |
| [15] |
HAMMERSCHMIDT C R, FITZGERALD W F, BALCOM P H, et al., 2008. Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor[J]. Marine Chemistry, 109(1): 165-182.
DOI URL |
| [16] |
HSU-KIM H, KUCHARZYK K H, ZHANG T, et al., 2013. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: A critical review[J]. Environmental Science & Technology, 47(6): 2441-2456.
DOI URL |
| [17] |
HUANG Y, WANG M, LI Z, et al., 2019. In situ remediation of mercury-contaminated soil using thiol-functionalized graphene oxide/Fe-Mn composite[J]. Journal of Hazardous Materials, 373: 783-790.
DOI PMID |
| [18] |
JEREMIASON J D, ENGSTROM D R, SWAIN E B, et al., 2006. Sulfate addition increases methylmercury production in an experimental wetland[J]. Environmental Science & Technology, 40(12): 3800-3806.
DOI URL |
| [19] |
JOHNSON W P, SWANSON N, BLACK B, et al., 2015. Total- and methyl-mercury concentrations and methylation rates across the freshwater to hypersaline continuum of the Great Salt Lake, Utah, USA[J]. The Science of the Total Environment, 511: 489-500.
DOI PMID |
| [20] |
JONSSON S, SKYLLBERG U, NILSSON M B, et al., 2012. Mercury methylation rates for geochemically relevant Hg(II) species in sediments[J]. Environmental Science & Technology, 46(21): 11653-11659.
DOI URL |
| [21] |
KERIN E J, GILMOUR C C, RODEN E, et al., 2006. Mercury methylation by dissimilatory iron-reducing bacteria[J]. Applied and Environmental Microbiology, 72(12): 7919-7921.
PMID |
| [22] |
KONDO R, PURDY K J, SILVA S DE Q, et al., 2007. Spatial dynamics of sulphate-reducing bacterial compositions in sediment along a salinity gradient in a UK Estuary[J]. Microbes and Environments, 22(1): 11-19.
DOI URL |
| [23] |
LELOUP J, PETIT F, BOUST D, et al., 2005. Dynamics of sulfate-reducing microorganisms (dsrAB genes) in two contrasting mudflats of the Seine Estuary (France)[J]. Microbial Ecology, 50(3): 307-314.
PMID |
| [24] |
LI H, ZHENG D M, YANG J S, et al., 2019. Salinity and redox conditions affect the methyl mercury formation in sediment of Suaeda heteroptera wetlands of Liaoning province, Northeast China[J]. Marine Pollution Bulletin, 142: 537-543.
DOI PMID |
| [25] |
MA M, DU H, WANG D, 2019. Mercury methylation by anaerobic microorganisms: A review[J]. Critical Reviews in Environmental Science and Technology, 49(20): 1893-1936.
DOI |
| [26] | MAZRUI N M, SEELEN E, KING’ONDU C K, et al., 2018. The precipitation, growth and stability of mercury sulfide nanoparticles formed in the presence of marine dissolved organic matter[J]. Environmental Science: Processes & Impacts, 20(4): 642-656. |
| [27] |
MEHROTRA A S, SEDLAK D L, 2005. Decrease in net mercury methylation rates following iron amendment to anoxic wetland sediment slurries[J]. Environmental Science & Technology, 39(8): 2564-2570.
DOI URL |
| [28] |
MOREND F N, ANDERSON CHRISTOPHER W N, STEWART R B, et al., 2005. Induced plant uptake and transport of mercury in the presence of Sulphur-containing ligands and humic acid[J]. New Phytologist, 166(2): 445-454.
PMID |
| [29] |
OREM W, GILMOUR C, AXELRAD D, et al., 2011. Sulfur in the South Florida ecosystem: distribution, sources, biogeochemistry, impacts, and management for restoration[J]. Critical Reviews in Environmental Science and Technology, 41(sup1): 249-288.
DOI URL |
| [30] | OREN A, 2016. Life in Hypersaline Environments[M]. Cham: Springer International Publishing: 301-339. |
| [31] |
PARKS J M, JOHS A, PODAR M, et al., 2013. The genetic basis for bacterial mercury methylation[J]. Science, 339(6125): 1332-1335.
DOI PMID |
| [32] | PODAR M, GILMOUR C C, BRANDT C C, et al., 2015. Global prevalence and distribution of genes and microorganisms involved in mercury methylation[J]. Science Advances, 1(9): e1500675. |
| [33] |
RAVICHANDRAN M, AIKEN G R, RYAN J N, et al., 1999. Inhibition of precipitation and aggregation of metacinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades[J]. Environmental Science & Technology, 33(9): 1418-1423.
DOI URL |
| [34] |
RILEY J P, TONGUDAI M, 1967. The major cation/chlorinity ratios in sea water[J]. Chemical Geology, 2: 263-269.
DOI URL |
| [35] |
RIVERA N A, BIPPUS P M, HSU-KIM H, 2019. Relative reactivity and bioavailability of mercury sorbed to or coprecipitated with aged iron sulfides[J]. Environmental Science & Technology, 53(13): 7391-7399.
DOI URL |
| [36] |
SHAO D D, KANG Y, WU S C, et al., 2012. Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments[J]. Science of The Total Environment, 424: 331-336.
DOI URL |
| [37] |
SINGH A K, HASNAIN S I, BANERJEE D K, 1999. Grain size and geochemical partitioning of heavy metals in sediments of the Damodar River-a tributary of the lower Ganga, India[J]. Environmental Geology, 39(1): 90-98.
DOI URL |
| [38] |
SKYLLBERG U, BLOOM P R, QIAN J, et al., 2006. Complexation of mercury(II) in soil organic matter: EXAFS evidence for linear Two-Coordination with reduced sulfur groups[J]. Environmental Science & Technology, 40(13): 4174-4180.
DOI URL |
| [39] |
SMYLIE M S, MCDONOUGH C J, REED L A, et al., 2016. Mercury bioaccumulation in an estuarine predator: Biotic factors, abiotic factors, and assessments of fish health[J]. Environmental Pollution, 214: 169-176.
DOI PMID |
| [40] |
STEFFAN R J, KORTHALS E T, WINFREY M R, 1988. Effects of acidification on mercury methylation, demethylation, and volatilization in sediments from an acid-susceptible lake[J]. Applied and Environmental Microbiology, 54(8): 2003-2009.
DOI PMID |
| [41] |
ULLRICH S M, TANTON T W, ABDRASHITOVA S A, 2001. Mercury in the aquatic environment: A review of factors affecting methylation[J]. Critical Reviews in Environmental Science and Technology, 31(3): 241-293.
DOI URL |
| [42] | WANG J, DAI J, CHEN G, et al., 2022. Role of sulfur biogeochemical cycle in mercury methylation in estuarine sediments: A review[J]. Journal of Hazardous Materials, 423: 126964. |
| [43] | WANG Y, LIU J, LIEM-NGUYEN V, et al., 2022. Binding strength of mercury (II) to different dissolved organic matter: The roles of DOM properties and sources[J]. Science of The Total Environment, 807: 150979. |
| [44] | YUAN K, CHEN X, CHEN P, et al., 2019. Mercury methylation-related microbes and genes in the sediments of the Pearl River Estuary and the South China Sea[J]. Ecotoxicology and Environmental Safety, 185: 109722. |
| [45] |
ZHANG L J, WU S, ZHAO L D, et al., 2019. Mercury sorption and desorption on Organo-Mineral particulates as a source for microbial methylation[J]. Environmental Science & Technology, 53(5): 2426-2433.
DOI URL |
| [46] |
ZHANG T, KUCHARZYK K H, KIM B, et al., 2014. Net methylation of mercury in estuarine sediment microcosms amended with dissolved, nanoparticulate, and microparticulate mercuric sulfides[J]. Environmental Science & Technology, 48(16): 9133-9141.
DOI URL |
| [47] | 杜红霞, IGARASHI Y, 王定勇, 等, 2014. 汞在微生物中的跨膜运输机制研究进展[J]. 微生物学报, 54(10): 1109-1115. |
| DU H X, IGARASHI Y, WANG D Y, et al., 2014. Transmembrane transport of inorganic mercury in microorganisms: A review[J]. Acta Microbiologica Sinica, 54(10): 1109-1115. | |
| [48] | 吉云芸, 杨麒弘, 张彤, 2020. 土壤和底泥间隙水中汞-硫-铁纳米颗粒物的形成条件、结构特征及其在甲基汞生物合成中的作用[J]. 环境化学, 39(1): 1-7. |
| JI Y Y, YANG Q H, ZHANG T, 2020. Formation and structure of mercury-sulfide-iron nanoparticles and their role in the microbial production of methylmercury in soil and sediment porewater[J]. Environmental Chemistry, 39(1): 1-7. | |
| [49] | 李航, 郑冬梅, 马欢驰, 2018. 辽河口湿地沉积物中汞含量变化特征的模拟研究[J]. 农业环境科学学报, 37(4): 774-779. |
| LI H, ZHENG D M, MA H C, 2018. Simulation of total mercury content variability in wetland sediments in the Liaohe Estuary[J]. Journal of Agro-Environment Science, 37(4): 774-779. | |
| [50] | 李新荣, 沈德中, 1999. 硫酸盐还原菌的生态特性及其应用[J]. 应用与环境生物学报, 5(Z1): 10-13. |
| LI X R, SHEN D Z, 1999. Ecological characters and application of sulfate-reducing bacteria[J]. Chinese Journal of Applied & Environmental Biology, 5(Z1): 10-13 | |
| [51] | 龙颂元, 2020. 微生物与环境因子对滨海湿地土壤甲基汞分布的影响[D]. 北京: 中国林业科学研究院: 7-9. |
| LONG S Y, 2020. Effects of microorganisms and environmental factors on the distribution of methylmercury in coastal wetland soil[D]. Beijing: Chinese Academy of Forestry, 7-9. | |
| [52] | 龙颂元, 张曼胤, 刘魏魏, 等, 2019. 互花米草入侵对滨海盐沼土壤甲基汞的影响[J]. 中国环境科学, 39(12): 5200-5209. |
| LONG S Y, ZHANG M Y, LIU W W, et al., 2019. Effects of Spartina alterniflora invasion on soil methylmercury in coastal salt marshes[J]. China Environmental Science, 39(12): 5200-5209. | |
| [53] | 俞建国, 周小红, 罗英, 2012. 高温催化氧化法测定土壤中溶解性有机碳[J]. 广州化工, 40(1): 85-87. |
| YU J G, ZHOU X H, LUO Y, 2012. Determination of dissolved organic carbon in soil by high temperature catalytic oxidation method[J]. Guangzhou Chemical Industry, 40(1): 85-87. | |
| [54] | 张玉, 贺惠, 米铁柱, 等, 2016. 东海海域表层沉积物中硫酸盐还原菌分布特征研究[J]. 中国环境科学, 36(12): 3750-3758. |
| ZHANG Y, HE H, MI T Z, et al., 2016. Distribution of sulfate-reducing bacteria in surface sediments from East China Sea[J]. China Environmental Science, 36(12): 3750-3758. | |
| [55] | 赵蕾, 2016. 汞矿区稻田土壤中汞的分布特征及甲基化/去甲基化速率研究[D]. 重庆: 西南大学: 69-70. |
| ZHAO L, 2016. Distribution patterns and methylation/demethylation rate of mercury in rice paddy in Hg mining area[D]. Chongqing: Southwest University: 69-70. | |
| [56] | 周心劝, 刘玉荣, 李晶, 等, 2018. 大兴安岭南瓮河湿地类型对土壤中甲基汞分布的影响[J]. 环境科学, 39(12): 5380-5486. |
| ZHOU X Q, LIU Y R, LI J, et al., 2018. Effects of wetland types on distribution of soil methylmercury based on the region of Nanweng River in the Greater Xing’an mountains[J]. Environmental Science, 39(12): 5380-5486. |
| [1] | 黄英梅, 钟松雄, 朱忆雯, 王向琴, 李芳柏. 单质硫抑制水稻植株甲基汞累积的效应与机制[J]. 生态环境学报, 2023, 32(6): 1115-1122. |
| [2] | 袁林江, 李梦博, 冷钢, 钟冰冰, 夏大朋, 王景华. 厌氧环境下硫酸盐还原与氨氧化的协同作用[J]. 生态环境学报, 2023, 32(1): 207-214. |
| [3] | 刘展航, 张树岩, 侯玉平, 朱书玉, 王立冬, 施欣悦, 李培广, 韩广轩, 谢宝华. 互花米草入侵对黄河口湿地土壤碳氮磷及其生态化学计量特征的影响[J]. 生态环境学报, 2022, 31(7): 1360-1369. |
| [4] | 柯丽娜, 徐佳慧, 王楠, 侯俊轩, 韩旭, 阴曙升. 基于遥感生态指数的滨海湿地生态质量变化评价——以辽东湾北部区为例[J]. 生态环境学报, 2022, 31(7): 1417-1424. |
| [5] | 刘志君, 崔丽娟, 李伟, 李晶, 雷茵茹, 朱怡诺, 王汝苗, 窦志国. 互花米草入侵对盐城滨海湿地nirS型反硝化细菌多样性及群落结构的影响[J]. 生态环境学报, 2022, 31(4): 704-714. |
| [6] | 胡瑞, 房焕英, 肖胜生, 段剑, 张杰, 刘洪光, 汤崇军. 南方红壤典型花岗岩侵蚀区主要治理模式的土壤碳汇效应[J]. 生态环境学报, 2021, 30(8): 1617-1626. |
| [7] | 蔡杨, 李伟, 左雪燕, 崔丽娟, 雷茵茹, 赵欣胜, 翟夏杰, 李晶, 潘旭. 盐城滨海湿地土壤多环芳烃分布特征及影响因素[J]. 生态环境学报, 2021, 30(6): 1249-1259. |
| [8] | 童辉, 乔江涛, 周继梅, 雷琴凯, 陈曼佳, 刘承帅. 硫酸盐还原菌介导针铁矿表面硫的转化及镉固定脱毒效应[J]. 生态环境学报, 2021, 30(5): 1069-1075. |
| [9] | 李侠, 兰建英, 蒋海明. 金属依赖型厌氧甲烷氧化研究进展[J]. 生态环境学报, 2021, 30(11): 2257-2266. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
