生态环境学报 ›› 2021, Vol. 30 ›› Issue (5): 1042-1050.DOI: 10.16258/j.cnki.1674-5906.2021.05.017
张晋龙1( ), 黄颖1, 吴丽芳1, 龚云辉1, 刘云根1,2,*(
), 黄颖1, 吴丽芳1, 龚云辉1, 刘云根1,2,*( ), 王妍1,2, 杨思林1
), 王妍1,2, 杨思林1
收稿日期:2020-10-09
出版日期:2021-05-18
发布日期:2021-08-06
通讯作者:
* 刘云根(1978年生),男,教授,博士,主要从事湿地生态和环境研究。E-mail:henryliu1008@163.com作者简介:张晋龙(1994年生),男,硕士研究生,主要从事湿地生态环境研究。E-mail:844014723@qq.com
基金资助:
ZHANG Jinlong1( ), HUANG Ying1, WU Lifang1, GONG Yunhui1, LIU Yungen1,2,*(
), HUANG Ying1, WU Lifang1, GONG Yunhui1, LIU Yungen1,2,*( ), WANG Yan1,2, YANG Silin1
), WANG Yan1,2, YANG Silin1
Received:2020-10-09
Online:2021-05-18
Published:2021-08-06
摘要:
为探究狭叶香蒲(Typha angustifolia L.)对砷(As)的耐受性和解毒机制,采用室内水培模拟实验,分析在不同As质量浓度(0、0.5、2、5、10 mg∙L-1)污染下,As在狭叶香蒲体内的富集、转运和亚细胞分布特征及对植物生长、生理生态特征的影响。结果表明,(1)狭叶香蒲株高、根长和生物量均随As胁迫浓度的增加呈先升高后下降的趋势,在5 mg∙L-1 As处理时达到最大值。(2)狭叶香蒲吸收的As主要富集在根部,占总富集量的52.92%—93.47%,富集系数和转移系数与As胁迫浓度呈负相关关系。As在根和叶的可溶性组分(F4)和细胞壁(F1)中的分布比例最高,在叶和根中F1、F4分配比例之和分别为64.48%—80.99%和82.21%—92.96%,但累积量分布极不均匀,主要分布在根的可溶性组分中,占植株总累积量的21.21%—67.17%,其次是根的细胞壁中,占19.74%—39.26%。(3)随着As胁迫浓度的增加,叶片超氧化物歧化酶(SOD)、过氧化物酶(POD)和过氧化氢酶(CAT)活性以及叶绿素含量和净光合速率均呈先上升后下降的趋势,叶绿素a与叶绿素b的比值则相反,As胁迫下丙二醛(MDA)含量为27.91—72.93 nmol∙g-1,为对照组的1.36—3.56倍。综合分析可知,狭叶香蒲通过减少As向地上部分的转移和细胞壁的固持、可溶性组分的钝化以及调节抗氧化酶活性等,降低As对狭叶香蒲的毒性,从而维持自身正常的生理状态。
中图分类号:
张晋龙, 黄颖, 吴丽芳, 龚云辉, 刘云根, 王妍, 杨思林. 砷胁迫对狭叶香蒲生理生态及砷亚细胞分布的影响[J]. 生态环境学报, 2021, 30(5): 1042-1050.
ZHANG Jinlong, HUANG Ying, WU Lifang, GONG Yunhui, LIU Yungen, WANG Yan, YANG Silin. As Subcellular Distribution and Physiological Response of Typha angustifolia L. to As Exposure[J]. Ecology and Environment, 2021, 30(5): 1042-1050.
| ρ(As)/ (mg∙L-1) | Plant height/ cm | root length/ cm | Leaf fresh weight plant/cm | Root fresh weight plant/cm |
|---|---|---|---|---|
| 0 | 99.33±2.49b | 9.33±0.94c | 21.05±2.93bc | 11.47±1.69b |
| 0.5 | 102.00±3.00b | 11.17±0.62ab | 20.00±1.40c | 16.79±1.32a |
| 2 | 113.33±5.73a | 12.17±0.85a | 25.63±2.10ab | 16.34±1.85a |
| 5 | 100.50±2.50b | 12.50±0.50a | 27.17±2.18a | 18.14±0.99a |
| 10 | 97.00±1.63b | 10.75±0.25bc | 22.01±2.59bc | 12.56±1.38b |
表1 狭叶香蒲生长特征对As胁迫的响应
Table 1 Response of Typha growth characteristics to As stress
| ρ(As)/ (mg∙L-1) | Plant height/ cm | root length/ cm | Leaf fresh weight plant/cm | Root fresh weight plant/cm |
|---|---|---|---|---|
| 0 | 99.33±2.49b | 9.33±0.94c | 21.05±2.93bc | 11.47±1.69b |
| 0.5 | 102.00±3.00b | 11.17±0.62ab | 20.00±1.40c | 16.79±1.32a |
| 2 | 113.33±5.73a | 12.17±0.85a | 25.63±2.10ab | 16.34±1.85a |
| 5 | 100.50±2.50b | 12.50±0.50a | 27.17±2.18a | 18.14±0.99a |
| 10 | 97.00±1.63b | 10.75±0.25bc | 22.01±2.59bc | 12.56±1.38b |
| Part | ρ(As)/ (mg∙L-1) | ω(As)/(mg∙kg-1) | Recovery rate/% | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |||
| Leaf | 0 | 0.13±0.01c | 0.09±0.01b | 0.09±0.02b | 0.22±0.05e | 90.62 |
| 0.5 | 0.33±0.04bc | 0.16±0.03b | 0.24±0.03a | 0.39±0.06d | 95.65 | |
| 2 | 0.62±0.09ab | 0.17±0.08ab | 0.23±0.01a | 0.82±0.01c | 102.86 | |
| 5 | 0.88±0.09a | 0.26±0.02a | 0.27±0.04a | 1.14±0.01b | 95.99 | |
| 10 | 0.95±0.02a | 0.27±0.01a | 0.28±0.03a | 1.39±0.14a | 93.18 | |
| Root | 0 | 0.77±0.10c | 0.15±0.05c | 0.13±0.02c | 0.55±0.05c | 109.86 |
| 0.5 | 5.33±0.36b | 1.62±0.13b | 0.56±0.08b | 4.73±0.35c | 103.86 | |
| 2 | 5.58±0.81b | 1.82±0.38b | 0.47±0.10b | 13.41±5.49b | 96.68 | |
| 5 | 7.35±1.28b | 1.95±0.48b | 0.74±0.20b | 19.59±2.99b | 89.81 | |
| 10 | 15.31±1.74a | 2.78±0.06a | 2.32±0.21a | 52.07±0.49a | 93.01 | |
表2 狭叶香蒲根和叶中As的亚细胞分布
Table 2 Subcellular distribution of As in roots and leaves of Typha
| Part | ρ(As)/ (mg∙L-1) | ω(As)/(mg∙kg-1) | Recovery rate/% | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |||
| Leaf | 0 | 0.13±0.01c | 0.09±0.01b | 0.09±0.02b | 0.22±0.05e | 90.62 |
| 0.5 | 0.33±0.04bc | 0.16±0.03b | 0.24±0.03a | 0.39±0.06d | 95.65 | |
| 2 | 0.62±0.09ab | 0.17±0.08ab | 0.23±0.01a | 0.82±0.01c | 102.86 | |
| 5 | 0.88±0.09a | 0.26±0.02a | 0.27±0.04a | 1.14±0.01b | 95.99 | |
| 10 | 0.95±0.02a | 0.27±0.01a | 0.28±0.03a | 1.39±0.14a | 93.18 | |
| Root | 0 | 0.77±0.10c | 0.15±0.05c | 0.13±0.02c | 0.55±0.05c | 109.86 |
| 0.5 | 5.33±0.36b | 1.62±0.13b | 0.56±0.08b | 4.73±0.35c | 103.86 | |
| 2 | 5.58±0.81b | 1.82±0.38b | 0.47±0.10b | 13.41±5.49b | 96.68 | |
| 5 | 7.35±1.28b | 1.95±0.48b | 0.74±0.20b | 19.59±2.99b | 89.81 | |
| 10 | 15.31±1.74a | 2.78±0.06a | 2.32±0.21a | 52.07±0.49a | 93.01 | |
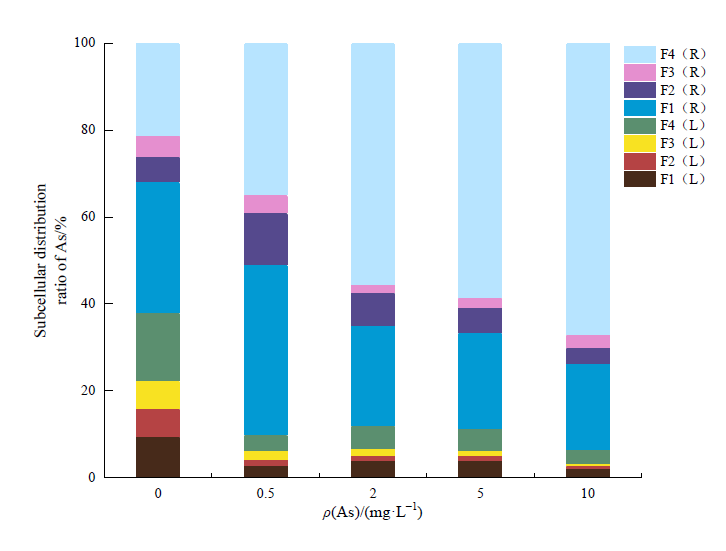
图6 不同As浓度下各亚细胞组分的As富集量占植株总As量的百分比L:叶。R:根
Fig. 6 Percentage of As enrichment of each subcellular component in total As content of plants under different As concentrationsL: Leaf。R: Root
| [1] |
ALI H M M, PERVEEN S, 2020. Effect of foliar applied triacontanol on wheat (Triticum aestivum L.) under arsenic stress: A study of changes in growth, yield and photosynthetic characteristics[J]. Physiology and Molecular Biology of Plants, DOI:10.1007/s12298-020-00831-0.
DOI |
| [2] | BAI J Y, FENG H Q, GUAN D D, et al., 2016. Extracellular ATP affects the copper-induced cell death and H2O2 production in tobacco (Nicotiana tabacum L.) cell-suspension cultures[J]. Journal of East China Normal University, 03(187): 107-119. |
| [3] | CALDELAS C, BORT J, FEBRERO A, 2012. Ultrastructure and subcellular distribution of Cr inIris pseudacorus L. using TEM and X-ray microanalysis[J]. Cell Biology&Toxicology, 28(1): 57-68. |
| [4] |
DING Y, DI X, NORTON G J, et al., 2020. Selenite Foliar Application Alleviates Arsenic Uptake, Accumulation, Migration and Increases Photosynthesis of Different Upland Rice Varieties[J]. International Journal of Environmental Research and Public Health, 17(10): 3621-3636.
DOI URL |
| [5] |
FENG RW, WANG X L, WEI C Y, et al., 2015. The Accumulation and Subcellular Distribution of Arsenic and Antimony in Four Fern Plants[J]. International Journal of Phytoremediation, 17(4): 348-354.
DOI URL |
| [6] |
FU X P, DOU C M, CHEN Y X, et al., 2011. Subcellular distribution and chemical forms of cadmium inPhytolacca americana L.[J]. Journal of Hazardous Materials, 186(1): 103-107.
DOI URL |
| [7] | GALBRAITH H, LEJEUNE K, LIPTON J, 2010. Metal and arsenic impacts to soils, vegetation communities and wildlife habitat in southwest Montana uplands contaminated by smelter emissions: I. Field evaluation[J]. Environmental Toxicology & Chemistry, 14(11): 1895-1903. |
| [8] |
GEFFARD A, SARTELET H, GARRIC J, et al., 2010. Subcellular compartmentalization of cadmium, nickel, and lead inGammarus fossarum: Comparison of methods[J]. Chemosphere, 78(7): 822-829.
DOI URL |
| [9] |
HALL J L, 2002. Cellular mechanisms for heavy metal detoxification and tolerance[J]. Journal of Experimental Botany, 53(366): 1-11.
DOI URL |
| [10] |
KOFRONOVA M, HRDINOVA A, MASKOVA P, et al., 2019. Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation[J]. Ecotoxicology and Environmental Safety, 174(6): 295-304.
DOI URL |
| [11] |
LI S, CHEN J R, ISLAM E, et al., 2016. Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (Phyllostachys pubescens) seedlings[J]. Chemosphere, 153(6): 107-114.
DOI URL |
| [12] |
MARQUES A P G C, RANGEL A O S S, CASTRO P M L, 2011. Remediation of Heavy Metal Contaminated Soils: An Overview of Site Remediation Techniques[J]. Critical Reviews in Environmental Science and Technology, 41(10): 879-914.
DOI URL |
| [13] |
MISHRA S, ALFELD M, SOBOTKA R, et al., 2016. Analysis of sublethal arsenic toxicity toCeratophyllum demersum: subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis[J]. Journal of experimental botany, 67(15): 4639-4646.
DOI URL |
| [14] |
MOBIN M, KHAN N A, 2007. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress[J]. Journal of Plant Physiology, 164(5): 601-610.
DOI URL |
| [15] |
PATRIZIA B, LETIZIA Z, ANGELO D P, et al., 2015. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance inArabidopsis [J]. Journal of Experimental Botany, 66(13): 3815-3830.
DOI URL |
| [16] |
PRAVEEN A, PANDEY C, MEHROTRA S, et al., 2019. Arsenic accumulation in Canna: Effect on antioxidative defense system[J]. Applied Geochemistry, DOI:10.1016/j.apgeochem.2019.06.001.
DOI |
| [17] |
RODRIGUEZ-LADO L, SUN G, BERG M, et al., 2013. Groundwater Arsenic Contamination Throughout China[J]. Science, 341(6148): 866-868.
DOI URL |
| [18] |
SHARMA S S, DIETZ K J, MIMURA T, 2016. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants[J]. Plant Cell and Environment, 39(5): 1112-1126.
DOI URL |
| [19] | TAUQEER H M, ALI S, RIZWAN M, et al., 2016. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response[J]. Ecotoxicology & Environmental Safety, 126(4): 138-146. |
| [20] |
WANG Y, SHEN H, XU L, et al., 2015. Transport, ultrastructural localization, and distribution of chemical forms of lead in radish (Raphanus sativus L.)[J]. Frontiers in Plant Science, DOI:10.3389/fpls.2015.00293.
DOI |
| [21] | XIN J L, HUANG B F, 2014. Subcellular Distribution and Chemical Forms of Cadmium in Two Hot Pepper Cultivars Differing in Cadmium Accumulation[J]. Journal of Agricultural & Food Chemistry, 62(2): 508-515. |
| [22] |
XIN J, ZHAO X H, TAN Q L, et al., 2017. Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.)[J]. Ecotoxicology and Environmental Safety, 145(11): 258-265.
DOI URL |
| [23] |
ZHANG F Q, WANG Y S, LOU Z P, et al., 2007. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel andBruguiera gymnorrhiza)[J]. Chemosphere, 67(1): 44-50.
DOI URL |
| [24] |
ZHANG H Z, GUO Q J, YANG J X, et al., 2015. Subcellular cadmium distribution and antioxidant enzymatic activities in the leaves of two castor (Ricinus communis L.) cultivars exhibit differences in Cd accumulation[J]. Ecotoxicology and environmental safety, 120: 184-192.
DOI URL |
| [25] | 陈璐, 米艳华, 万小铭, 等, 2015. 砷在药用植物三七根部组织及其亚细胞分布特征[J]. 植物学报, 50(5): 591-597. |
| CHE L, MI Y H, WAN X M, et al., 2015. Distribution Characteristics of Arsenic in Medicinal PlantsPanax notoginseng's Taproots Tissue and Subcellular Components[J]. Chinese Bulletin of Botany, 50(5): 591-597. | |
| [26] | 陈天, 包宁颖, 杜崇宣, 等, 2020. 重金属污染河流生态修复区挺水植物对重金属的吸收特性[J]. 环境科学研究, 33(9): 2110-2117. |
| CHE T, BAO N Y, DU C X, et al., 2020. Absorption Characteristics of Heavy Metals by Emergent Plants from Polluted River in Ecological Restoration Areas[J]. Research of Environmental Sciences, 33(9): 2110-2117. | |
| [27] | 陈天, 刘云根, 王妍, 等, 2019. 外源磷对砷胁迫下挺水植物抗氧化酶系统的影响[J]. 江苏农业学报, 35(5): 1040-1046. |
| CHEN T, LIU Y G, WANG Y, et al., 2019. Effects of exogenous phosphorus on antioxidant enzyme system of emergent plants under arsenic stress[J]. Jiangsu Journal of Agricultural Sciences, 35(5): 1040-1046. | |
| [28] | 陈同斌, 阎秀兰, 廖晓勇, 等, 2005. 蜈蚣草中砷的亚细胞分布与区隔化作用[J]. 科学通报, 50(24): 2739-2744. |
| CHENG T B, YAN X L, LIAO X Y, et al., 2005. Subcellular distribution and isolation of Arsenic inPteris vittata L.[J]. Chinese Science Bulletin, 50(24): 2739-2744. | |
| [29] | GERALD Z, 2015. 烟草砷吸收、亚细胞分布及形态的基因型差异与磷酸盐缓解砷毒害的机理研究[D]. 杭州: 浙江大学: 31. |
| GERALD Z, 2015. Genotypic difference in arsenic uptake, subcellul distribution and speciation in Tobacco and the possible role of phosphate alleviating arsenic toxicity[D]. Hangzhou: Zhejiang Uniwersity: 31. | |
| [30] | 郝玉波, 刘华琳, 慈晓科, 等, 2010. 砷对玉米生长、抗氧化系统及离子分布的影响[J]. 应用生态学报, 21(12): 3183-3 190. |
| HAO Y B, LIU H L, CI X K, et al., 2010. Effects of arsenic on maize growth,antioxidant system, and ion distribution[J]. Chinese Journal of Applied Ecology, 21(12): 3183-3190. | |
| [31] | 胡拥军, 王海娟, 王宏镔, 等, 2015. 砷胁迫下不同砷富集能力植物内源生长素与抗氧化酶的关系[J]. 生态学报, 35(10): 3214-3224. |
| HU Y J, WANG H J, WANG H B, et al., 2015. The relationship between endogenous auxin and antioxidative enzymes in two plants with different arsenic-accumulative ability under arsenic stress[J]. Acta Ecologica Sinica, 35(10): 3214-3224. | |
| [32] | 廖晓勇, 谢华, 陈同斌, 等, 2007. 蜈蚣草的超微结构和砷、钙的亚细胞分布[J]. 植物营养与肥料学报, 13(2): 305-312. |
| LIAO X Y, XIE H, CHENG T B, et al., 2007. Ultrastructure and subcellular distributions of arsenic and calcium inPteris vittata L.[J]. Journal of Plant Nutrition and Fertilizers, 13(2): 305-312. | |
| [33] | 刘全吉, 孙学成, 胡承孝, 等, 2009. 砷对小麦生长和光合作用特性的影响[J]. 生态学报, 29(2): 854-859. |
| LIU J Q, SUN X C, HU C X, et al., 2009. Growth and photosynthesis characteristics of wheat (Triticum aestivum L.) under arsenic stress condition[J]. Acta Ecologica Sinica, 29(2): 854-859. | |
| [34] | 罗洁文, 李莹, 苏烁烁, 等, 2016. 类芦根系抗氧化酶和植物螯合肽对Cd、Pb胁迫的应答[J]. 生态环境学报, 25(6): 1047-1053. |
| LUO J W, LI Y, SU S S, et al., 2016. Response of Antioxidant Enzymes and PCs in Root ofNeyraudia reynaudiana to Cd, Pb Stress[J]. Ecology and Environmental Sciences, 25(6): 1047-1053. | |
| [35] | 任伟, 倪大伟, 刘云根, 等, 2019. 砷污染生境下挺水植物香蒲对砷的积累与迁移特性[J]. 环境科学研究, 32(5): 848-856. |
| REN W, NI D W, LIU Y G, et al., 2019. Accumulation and Transportation of Arsenic to wetland PlantTypha angustifolia L. in the Herbaceous Plants Grown in Arsenic-Contaminated Habitat[J]. Research of Environmental Sciences, 32(5): 848-856. | |
| [36] | 赛闹汪青, 冉瑞兰, 张牡丹, 等, 2019. 铜胁迫对黄芪幼苗的生理学毒性与凹凸棒黏土的缓解作用[J]. 中国环境科学, 39(12): 5273-5284. |
| SAI N W Q, RAN R L, ZHANG M D, et al., 2019. Physiological toxicity of copper stress onAstragalus membranaceus seedlings and mitigation of attapulgite clay[J]. China Environmental Science, 39(12): 5273-5284. | |
| [37] | 尚德荣, 张继红, 赵艳芳, 等, 2013. 条斑紫菜中砷的亚细胞分布及其解毒机制的研究[J]. 分析化学, 41(11): 1647-1652. |
| SHANG D R, ZHANG J H, ZHAO Y F, et al., 2013. The Subcellular Fate of Phosphorus and Calcium in the SeaweedPorphyra yezoensis and Its Relationship with the Arsenic Accumulation[J]. Chinese Journal of Analytical Chemistry, 41(11): 1647-1652. | |
| [38] | 汪良驹, 刘友良, 1998. 植物细胞中的液泡及其生理功能[J]. 植物生理学通讯, 34(5): 394-400. |
| WANG L J, LIU Y L, 1998. Vacuoles of Plant Cells and Their Physiological Functions[J]. Plant Physiology Journal, 34(5): 394-400. | |
| [39] | 吴敏兰, 李荭荭, 贾洋洋, 等, 2015. 砷胁迫对不同烟草品种光合色素和叶绿素荧光特性的影响[J]. 生态毒理学报, 10(3): 216-223. |
| WU M L, LI H H, JIA Y Y, et al., 2015. Influence of Arsenic Stress on the Photosynthetic Pigments and Chlorophyll Fluorescence Characteristics of Different Tobacco Cultivars[J]. Asian Journal of Ecotoxicology, 10(3): 216-223. | |
| [40] | 易心钰, 2018. 蓖麻对铅锌胁迫的响应及其机制研究[D]. 长沙: 中南林业科技大学: 65. |
| YI X Y, 2018. Study on the responses of Ricinus communis L. \nto Lead and Zinc stress and their mechanisms[D]. Changsha: Central South University of Forestry & Technology: 65. | |
| [41] | 张永志, 赵首萍, 徐明飞, 等, 2015. 不同蒸腾作用对番茄幼苗吸收Pb、Cd的影响[J]. 生态环境学报, 18(2): 515-518. |
| ZHANG Y Z, ZHAO S P, XU M F, et al., 2015. Effect of transpiration rates on the absorption of Pb and Cd in seedling of tomato[J]. Ecology and Environmental Sciences, 18(2): 515-518. | |
| [42] | 郑国锠, 2000. 细胞生物学[M].第2版. 北京: 高等教育出版社: 127. |
| ZHENG G C, 2000. Cell Biology[M].SecondEdition.Beijing: Higher Education Press: 127. |
| [1] | 杨宇, 邓仁健, 隆佩, 黄中杰, 任伯帜, 王政华. 砷氧化菌Pseudomonas sp. AO-1的分离鉴定及其对As(Ⅲ)的氧化性能研究[J]. 生态环境学报, 2023, 32(3): 619-626. |
| [2] | 尹浩均, 龙明亮, 刘维, 倪春林, 李芳柏, 吴云当. 溶氧浓度调控嗜水气单胞菌的砷还原:效应与机制[J]. 生态环境学报, 2023, 32(2): 381-387. |
| [3] | 高鹏, 高品, 孙蔚旻, 孔天乐, 黄端仪, 刘华清, 孙晓旭. 蜈蚣草根际及内生微生物群落对砷污染胁迫的响应机制研究[J]. 生态环境学报, 2022, 31(6): 1225-1234. |
| [4] | 徐梅华, 顾明华, 王骋臻, 雷静, 韦燕燕, 沈方科. 锰对土壤砷形态转化及水稻吸收砷的影响[J]. 生态环境学报, 2022, 31(4): 802-813. |
| [5] | 曾民, 陈佳, 李娥贤, 殷富有, 王玲仙, 曾黎琼, 郭蓉. 元江普通野生稻后代镉分布特点及镉积累动态变化规律[J]. 生态环境学报, 2022, 31(3): 565-571. |
| [6] | 刘畅, 罗艳丽, 刘晨通, 郑玉红, 晁博, 董乐乐. 奎屯河下游区域地下水和农田土壤砷的空间分布特征[J]. 生态环境学报, 2022, 31(10): 2070-2078. |
| [7] | 许冬雪, 李兴, 王勇, 勾芒芒. 冰封期乌梁素海不同形态氮、磷和叶绿素a的空间分布特征及其响应关系[J]. 生态环境学报, 2021, 30(9): 1855-1864. |
| [8] | 袁伟皓, 王华, 夏玉宝, 曾一川, 邓燕青, 李媛媛, 张心悦. 基于GAM模型的鄱阳湖叶绿素a与水质因子相关性分析[J]. 生态环境学报, 2021, 30(8): 1716-1723. |
| [9] | 丛超, 杨宁柯, 王海娟, 王宏镔. 吲哚乙酸和激动素配合施用提高蜈蚣草和龙葵对砷、镉富集的田间试验[J]. 生态环境学报, 2021, 30(6): 1299-1309. |
| [10] | 张鹏, 刘玮, 王铁杆, 钟晨辉, 陶月良. 无机砷短期胁迫对铜藻幼苗氧化损伤、抗氧化酶及抗氧化物的影响[J]. 生态环境学报, 2021, 30(5): 1034-1041. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
