生态环境学报 ›› 2022, Vol. 31 ›› Issue (1): 205-214.DOI: 10.16258/j.cnki.1674-5906.2022.01.023
• 综述 •
上一篇
吴洁婷1( ), 赵若帆1, 包红旭1, 张营1, 赵磊2, 许琪1, 陈忠林1, 徐丽丽1, 张驰1, 许海萍3, 马放2,*(
), 赵若帆1, 包红旭1, 张营1, 赵磊2, 许琪1, 陈忠林1, 徐丽丽1, 张驰1, 许海萍3, 马放2,*( )
)
收稿日期:2021-07-22
出版日期:2022-01-18
发布日期:2022-03-10
通讯作者:
*马放,男,教授,博士。E-mail: mafang1963@163.com作者简介:吴洁婷(1987年生),女,副教授,博士,主要研究方向为污染场地生物修复、菌根际微界面效应及分子生物学机制及重金属生物体迁移转化趋向调控机理。E-mail: laurelwuchina@163.com
基金资助:
WU Jieting1( ), ZHAO Ruofan1, BAO Hongxu1, ZHANG Ying1, ZHAO Lei2, XU Qi1, CHEN Zhonglin1, XU Lili1, ZHANG Chi1, XU Haiping3, MA Fang2,*(
), ZHAO Ruofan1, BAO Hongxu1, ZHANG Ying1, ZHAO Lei2, XU Qi1, CHEN Zhonglin1, XU Lili1, ZHANG Chi1, XU Haiping3, MA Fang2,*( )
)
Received:2021-07-22
Online:2022-01-18
Published:2022-03-10
摘要:
多环芳烃(PAHs)是普遍存在于环境中具有强烈毒性、致突变性和致癌性的难降解有机物,可造成严重的环境污染。由于低水溶性而导致的低生物可利用率是限制PAHs微生物降解的主要因素。生物表面活性剂鼠李糖脂由于在形成胶束后能够大幅提高PAHs的表观溶解度,且毒性低、无二次污染,因而在PAHs微生物降解的研究中得到广泛关注。目前关于鼠李糖脂强化PAHs微生物降解的研究主要集中于其强化效果,而对其强化机制的研究仍不够深入。该文基于鼠李糖脂的性质及铜绿假单胞菌(Pseudomonas aeruginosa)的鼠李糖脂生物合成及调控,从鼠李糖脂提高PAHs溶解度、强化胶束传质、提高细胞表面疏水性、降低细胞表面Zeta电位、提高细胞膜通透性等方面综述其在强化PAHs微生物降解机制方面的最新研究进展,并总结了温度、pH、浓度和离子强度等环境因素对强化效果的影响。在此基础上,提出未来需要进一步探索鼠李糖脂生物可降解性与强化降解效果之间的平衡关系,明确pH影响PAHs溶解度的机理,并从基因、转录、蛋白和代谢水平对鼠李糖脂作用前后降解菌内参与调控菌体细胞表面疏水性(CSH)和膜通透性的相关基因的表达差异进行分析,阐释相关强化机制的深层机理,寻求降解过程中菌体最佳CSH和膜通透性,找寻使降解菌达到降解最佳状态的基因调控手段,为进一步深入研究鼠李糖脂的强化机制提供理论支撑。
中图分类号:
吴洁婷, 赵若帆, 包红旭, 张营, 赵磊, 许琪, 陈忠林, 徐丽丽, 张驰, 许海萍, 马放. 鼠李糖脂强化多环芳烃微生物修复的研究进展[J]. 生态环境学报, 2022, 31(1): 205-214.
WU Jieting, ZHAO Ruofan, BAO Hongxu, ZHANG Ying, ZHAO Lei, XU Qi, CHEN Zhonglin, XU Lili, ZHANG Chi, XU Haiping, MA Fang. Research Progress on the Enhanced Microbial Remediation of Polycyclic Aromatic Hydrocarbons with Rhamnolipids[J]. Ecology and Environment, 2022, 31(1): 205-214.
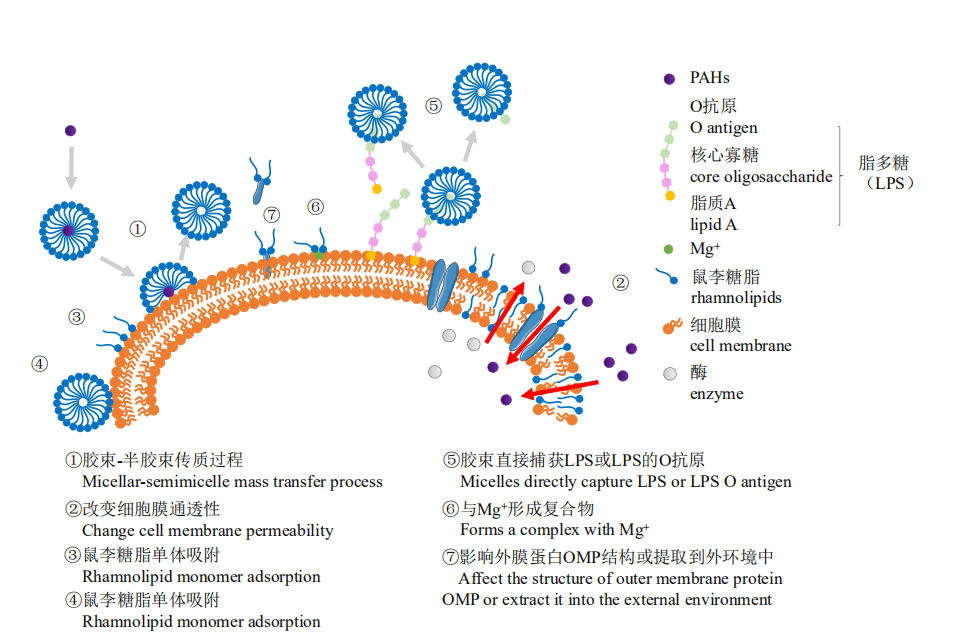
图3 鼠李糖脂对微生物特性及PAHs降解的影响过程 图中①过程为鼠李糖脂的传质强化过程;②③过程为鼠李糖脂在细胞表面吸附过程;④⑤⑥过程为鼠李糖脂促进LPS释放的3种可能的机制;⑦过程为鼠李糖脂改变细胞膜通透性的过程
Figure 3 Influence process of rhamnolipid on microbial properties and PAHs degradation
| [1] | ABDEL-MAWGOUD A M, HAUSMANN R, LéPINE F, et al., 2011. Rhamnolipids: Detection, analysis, biosynthesis, genetic regulation, and bioengineering of production[J]. Biosurfactants: 13-55. |
| [2] |
ABDEL-MAWGOUD A M, LEPINE F, DEZIEL E, 2010. Rhamnolipids: diversity of structures, microbial origins and roles[J]. Applied Microbiology and Biotechnology, 86(5): 1323-1336.
DOI URL |
| [3] |
ABOUSEOUD M, YATAGHENE A, AMRANE A, et al., 2010. Effect of pH and salinity on the emulsifying capacity and naphthalene solubility of a biosurfactant produced by Pseudomonas fluorescens[J]. Journal of Hazardous Materials, 180(1-3): 131-136.
DOI URL |
| [4] |
AGUIRRE-RAMÍREZ, MEDINA G, GONZÁLEZ-VALDEZ A, et al., 2012. The Pseudomonas aeruginosa rmlBDAC operon, encoding dTDP-L-rhamnose biosynthetic enzymes, is regulated by the quorum-sensing transcriptional regulator RhlR and the alternative sigma factor σS[J]. Microbiology, 158(Pt 4): 908-916.
DOI URL |
| [5] |
AHMAD Z, ZHANG X, IMRAN M, et al., 2021. Production, functional stability, and effect of rhamnolipid biosurfactant from Klebsiella sp. on phenanthrene degradation in various medium systems[J]. Ecotoxicology and Environmental Safety, DOI: 10.1016/j.ecoenv.2020.111514.
DOI |
| [6] |
AKBARI A, KASPRZYK A, GALVEZ R, et al., 2021. A rhamnolipid biosurfactant increased bacterial population size but hindered hydrocarbon biodegradation in weathered contaminated soils[J]. Science of the Total Environment, DOI: 10.1016/j.scitotenv.2021.145441.
DOI |
| [7] |
ALKHALAF S A, RAMADAN A R, OBUEKWE C, et al., 2021. Heavy vacuum gas oil upregulates the rhamnosyltransferases and quorum sensing cascades of rhamnolipids biosynthesis in Pseudomonas sp. AK6U[J]. Molecules, DOI: 10.3390/molecules26144122.
DOI |
| [8] |
AL-TAHHAN R A, SANDRIN T R, BODOUR A A, et al., 2000. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: Effect on cell surface properties and interaction with hydrophobic substrates[J]. Applied and Environmental Microbiology, 66(8): 3262-3268.
DOI URL |
| [9] |
ANDERSEN K K, OTZEN D E, 2014. Folding of outer membrane protein A in the anionic biosurfactant rhamnolipid[J]. FEBS Letters, 588(10): 1955-1960.
DOI URL |
| [10] |
BHATTACHARJEE A, NUSCA T D, HOCHBAUM A I, 2016. Rhamnolipids mediate an interspecies biofilm dispersal signaling pathway[J]. ACS Chemical Biology, 11(11): 3068-3076.
DOI URL |
| [11] |
BONDARENKO O, RAHMAN P K, RAHMAN T J, et al., 2010. Effects of rhamnolipids from Pseudomonas aeruginosa DS10-129 on luminescent bacteria: Toxicity and modulation of cadmium bioavailability[J]. Microbial Ecology, 59(3): 588-600.
DOI URL |
| [12] |
BRINDHADEVI K, LEWISOSCAR F, MYLONAKIS E, et al., 2020. Biofilm and quorum sensing mediated pathogenicity in Pseudomonas aeruginosa [J]. Process Biochemistry, 96: 49-57.
DOI URL |
| [13] |
CHONG H Q, LI Q X, 2017. Microbial production of rhamnolipids: opportunities, challenges and strategies[J]. Microbial Cell Factories, 16(1): 137.
DOI URL |
| [14] |
CRODA-GARCÍA G, GROSSO-BECRRA V, GONZALEZ-VALDEZ A, et al., 2011. Transcriptional regulation of Pseudomonas aeruginosa rhlR: role of the CRP orthologue Vfr (virulence factor regulator) and quorum-sensing regulators LasR and RhlR[J]. Microbiology, 157(9): 2545-2555.
DOI URL |
| [15] |
DOBLER L, VILELA L F, ALMEIDA R V, et al., 2016. Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting[J]. New Biotechnology, 33(1): 123-135.
DOI URL |
| [16] |
DUSANE D H, ZINJARDE S S, VENUGOPALAN V P, et al., 2010. Quorum sensing: Implications on rhamnolipid biosurfactant production[J]. Biotechnology and Genetic Engineering Reviews, 27(1): 159-184.
DOI URL |
| [17] |
ERAQI W, YASSIN A S, ALI A E, et al., 2016. Utilization of crude glycerol as a substrate for the production of rhamnolipid by Pseudomonas aeruginosa [J]. Biotechnology Research International, DOI: 10.1155/2016/3464509.
DOI |
| [18] |
FRANK N, LINER A, WINKELMANN M, et al., 2010. Degradation of selected (bio-)surfactants by bacterial cultures monitored by calorimetric methods[J]. Biodegradation, 21(2): 179-191.
DOI URL |
| [19] | GHOSH I, MUKHERJI S, 2016. Diverse effect of surfactants on pyrene biodegradation by a Pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction[J]. International Biodeterioration & Biodegradation, 108: 67-75. |
| [20] |
GROSSMAN S, SOUKARIEH F, RICHARDSON W, et al., 2020. Novel quinazolinone inhibitors of the Pseudomonas aeruginosa quorum sensing transcriptional regulator PqsR[J]. European Journal of Medicinal Chemistry, DOI: 10.1016/j.ejmech.2020.112778.
DOI |
| [21] |
HERZOG M, TISO T, BLANK L M, et al., 2020. Interaction of rhamnolipids with model biomembranes of varying complexity[J]. Biochimica et Biophysica Acta - Biomembranes, 1862(11): 183431.
DOI URL |
| [22] |
JAHAN R, BODRATTI A M, TSIANOU M, et al., 2020. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications[J]. Advances in Colloid and Interface Science, DOI: 10.1016/j.cis.2019.102061.
DOI |
| [23] | KACZOREK E, 2012. Effect of external addition of rhamnolipids biosurfactant on the modification of gram positive and gram negative bacteria cell surfaces during biodegradation of hydrocarbon fuel contamination[J]. Polish Journal of Environmental Studies, 21(4): 901-909. |
| [24] |
KACZOREK E, PACHOLAK A, ZDARTA A, et al., 2018. The impact of biosurfactants on microbial cell properties leading to hydrocarbon Bioavailability Increase[J]. Colloids and Interfaces, 2(3): 35-57.
DOI URL |
| [25] |
KACZOREK E, URBANOWICZ M, OLSZANOWSKI A, 2010. The influence of surfactants on cell surface properties of Aeromonas hydrophila during diesel oil biodegradation[J]. Colloids and Surfaces B: Biointerfaces, 81(1): 363-368.
DOI URL |
| [26] |
KARIMINIK A, BASERI-SALEHI M, KHEIRKHAH B, 2017. Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article[J]. Immunology Letters, 190: 1-6.
DOI URL |
| [27] |
KARLAPUDI A P, VENKATESWARULU T C, TAMMINEEDI J, et al., 2018. Role of biosurfactants in bioremediation of oil pollution-a review[J]. Petroleum, 4(3): 241-249.
DOI URL |
| [28] |
KIM C H, LEE D W, HEO Y M, et al., 2019. Desorption and solubilization of anthracene by a rhamnolipid biosurfactant from Rhodococcus fascians [J]. Water Environment Research, 91(8): 739-747.
DOI URL |
| [29] |
KIM L H, JUNG Y, YU H W, et al., 2015. Physicochemical interactions between rhamnolipids and Pseudomonas aeruginosa biofilm layers[J]. Environmental Science & Technology, 49(6): 3718-3726.
DOI URL |
| [30] |
KUMARI S, MANGWANI N, DAS S, 2016. Synergistic effect of quorum sensing genes in biofilm development and PAHs degradation by a marine bacterium[J]. Bioengineered, 7(3): 205-211.
DOI URL |
| [31] |
LAMICHHANE S, BAL KRISHNA K C, SARUKKALIGE R, 2017. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review[J]. Journal of Environmental Management, 199: 46-61.
DOI URL |
| [32] |
LI J L, CHEN B H, 2009. Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons[J]. Materials, 2(1): 76-94.
DOI URL |
| [33] |
LI Q X, 2017. Rhamnolipid synthesis and production with diverse resources[J]. Frontiers of Chemical Science and Engineering, 11(1): 27-36.
DOI URL |
| [34] |
LI S D, PI Y R, BAO M T, et al., 2015. Effect of rhamnolipid biosurfactant on solubilization of polycyclic aromatic hydrocarbons[J]. Marine Pollution Bulletin, 101(1): 219-225.
DOI URL |
| [35] |
LIANG X J, GUO C L, WEI Y F, et al., 2016. Cosolubilization synergism occurrence in codesorption of PAH mixtures during surfactant-enhanced remediation of contaminated soil[J]. Chemosphere, 144: 583-590.
DOI URL |
| [36] |
LIN W J, LIU S S, TONG L, et al., 2017. Effects of rhamnolipids on the cell surface characteristics of Sphingomonas sp. GY2B and the biodegradation of phenanthrene[J]. RSC Advances, 7(39): 24321-24330.
DOI URL |
| [37] | LIU G S, ZHONG H, YANG X, et al., 2018. Advances in applications of rhamnolipids biosurfactant in environmental remediation: A review[J]. Biofuels and Environmental Biotechnology, 115(4): 796-814. |
| [38] |
LIU J F, WANG Y R, LI H F, 2020. Synergistic solubilization of phenanthrene by mixed micelles composed of biosurfactants and a conventional non-Ionic surfactant[J]. Molecules, 25(18): 4327.
DOI URL |
| [39] |
LIU Y, MA X L, ZENG G M, et al., 2014. Role of low-concentration monorhamnolipid in cell surface hydrophobicity of Pseudomonas aeruginosa: adsorption or lipopolysaccharide content variation[J]. Applied Microbiology and Biotechnology, 98(24): 10231-10241.
DOI URL |
| [40] |
LIU Z F, ZENG G M, ZHONG GH, et al., 2011. Effect of saponins on cell surface properties of Penicillium simplicissimum: Performance on adsorption of cadmium(II)[J]. Colloids and Surfaces B: Biointerfaces, 86(2): 364-369.
DOI URL |
| [41] |
LIU Z F, ZENG Z T, ZENG G M, et al., 2012. Influence of rhamnolipids and Triton X-100 on adsorption of phenol by Penicillium simplicissimum [J]. Bioresource Technology, 110: 468-473.
DOI URL |
| [42] |
LOVAGLIO R B, SILVA V L, FERREIRA H, et al., 2015. Rhamnolipids know-how: Looking for strategies for its industrial dissemination[J]. Biotechnology Advances, 33(8): 1715-1726.
DOI URL |
| [43] |
LU H N, WANG W, LI F, et al., 2019. Mixed-surfactant-enhanced phytoremediation of PAHs in soil: Bioavailability of PAHs and responses of microbial community structure[J]. Science of the Total Environment, 653: 658-666.
DOI URL |
| [44] |
MA Z, LIU J, DICK R P, et al., 2018. Rhamnolipid influences biosorption and biodegradation of phenanthrene by phenanthrene-degrading strain Pseudomonas sp. Ph6[J]. Environmental Pollution, 240: 359-367.
DOI URL |
| [45] |
MALGAONKAR A, NAIR M, 2019. Quorum sensing in Pseudomonas aeruginosa mediated by RhlR is regulated by a small RNA PhrD[J]. Scientific Reports, 9(1): 432.
DOI URL |
| [46] |
MARIAAMALRAJ S K, PASUMARTHI R, ACHARY A, et al., 2016. Effect of rhamnolipid on biodegradation of hydrocarbons in non-aqueous-phase liquid (NAPL)[J]. Bioremediation Journal, 20(3): 183-193.
DOI URL |
| [47] |
MOHAN P K, NAKHLA G, YANFUL E K, 2006. Biokinetics of biodegradation of surfactants under aerobic, anoxic and anaerobic conditions[J]. Water Research, 40(3): 533-540.
DOI URL |
| [48] |
MOHANTY S, MUKHERJI S, 2013. Surfactant aided biodegradation of NAPLs by Burkholderia multivorans: comparison between Triton X-100 and rhamnolipid JBR-515[J]. Colloids and Surfaces B: Biointerfaces, 102: 644-652.
DOI URL |
| [49] |
MUKHERJEE S, MOUSTAFA D, SMITH C D, et al., 2017. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer[J]. PLoS Pathog, 13(7): e1006504.
DOI URL |
| [50] |
MÜLLER M M, HAUSMANN R, 2011. Regulatory and metabolic network of rhamnolipid biosynthesis: traditional and advanced engineering towards biotechnological production[J]. Applied Microbiology and Biotechnology, 91(2): 251-264.
DOI URL |
| [51] |
ORTEGA-CALVO J J, TEJEDA-AGREDANO M C, JIMENEZ-SANCHEZ C, et al., 2013. Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation?[J]. Journal of Hazardous Materials, 261: 733-745.
DOI URL |
| [52] | PATOWARY R, PATOWARY K, KALITA M C, et al., 2018. Application of biosurfactant for enhancement of bioremediation process of crude oil contaminated soil[J]. International Biodeterioration & Biodegradation, 129: 50-60. |
| [53] |
PENG X, YUAN X Z, LIU H, et al., 2015. Degradation of polycyclic aromatic hydrocarbons (PAHs) by laccase in rhamnolipid reversed micellar system[J]. Applied Biochemistry and Biotechnology, 176(1): 45-55.
DOI URL |
| [54] |
PERFUMO A, BANAT I M, CANGANELLA F, et al., 2006. Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1[J]. Applied Microbiology and Biotechnology, 72(1): 132-138.
DOI URL |
| [55] |
PHULPOTO I A, WANG Y, QAZI M A, et al., 2021. Bioprospecting of rhamnolipids production and optimization by an oil-degrading Pseudomonas sp. S2WE isolated from freshwater lake[J]. Bioresource Technology, DOI: 10.1016/j.biortech.2020.124601.
DOI |
| [56] |
RAMOS D A SILVA A, MANRESA M A, et al., 2019. Rhamnolipids functionalized with basic amino acids: Synthesis, aggregation behavior, antibacterial activity and biodegradation studies[J]. Colloids and Surfaces B: Biointerfaces, 181: 234-243.
DOI URL |
| [57] |
RASHEED T, SHAFI S, BILAL M, et al., 2020. Surfactants-based remediation as an effective approach for removal of environmental pollutants: A review[J]. Journal of Molecular Liquids, DOI: 10.1016/j.molliq.2020.113960.
DOI |
| [58] |
RATHANKUMAR A K, SAIKIA K, PONNUSAMY S K, et al., 2020. Rhamnolipid-assisted mycoremediation of polycyclic aromatic hydrocarbons by Trametes hirsuta coupled with enhanced ligninolytic enzyme production[J]. Journal of the Air and Waste Management Association, 70(12): 1260-1267.
DOI URL |
| [59] |
REDDY P V, KAREGOUDAR T B, NAYAK A S, 2018. Enhanced utilization of fluorene by Paenibacillus sp. PRNK-6: Effect of rhamnolipid biosurfactant and synthetic surfactants[J]. Ecotoxicology and Environmental Safety, 151: 206-211.
DOI URL |
| [60] |
REIS R S, PEREIRA A G, NEVES B C, et al., 2011. Gene regulation of rhamnolipid production in Pseudomonas aeruginosa: A review[J]. Bioresource Technology, 102(11): 6377-6384.
DOI URL |
| [61] |
SAUMYEN G, JAFFÉ P, 1996. Bioavailability of hydrophobic compounds partitioned into the micellar phase of nonionic surfactants[J]. Environmental Science & Technology, 30(4): 1382-1391.
DOI URL |
| [62] |
SHAO B B, LIU Z F, ZHONG H, et al., 2017. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review[J]. Microbiological Research, 200: 33-44.
DOI URL |
| [63] |
SHATILA F, DIALLO M M, SAHAR U, et al., 2020. The effect of carbon, nitrogen and iron ions on mono-rhamnolipid production and rhamnolipid synthesis gene expression by Pseudomonas aeruginosa ATCC 15442[J]. Archives of Microbiology, 202(6): 1407-1417.
DOI URL |
| [64] |
SINGLETON D R, ADRION A C, AITKEN M D, 2016. Surfactant-induced bacterial community changes correlated with increased polycyclic aromatic hydrocarbon degradation in contaminated soil[J]. Applied Microbiology and Biotechnology, 100(23): 10165-10177.
DOI URL |
| [65] |
SMULEK W, ZDARTA A, GUZIK U, et al., 2015. Rahnella sp. strain EK12: Cell surface properties and diesel oil biodegradation after long-term contact with natural surfactants and diesel oil[J]. Microbiological Research, 176: 38-47.
DOI URL |
| [66] |
SOBERÓN-CHÁVEZ G, GONZÁLEZ-VALDEZ A, SOTO-ACEVES M P, et al., 2021. Rhamnolipids produced by Pseudomonas: from molecular genetics to the market[J]. Microb Biotechnol, 14(1): 136-146.
DOI URL |
| [67] |
SOBERÓN-CHÁVEZ G, LÉPINE F, DÉZIEL E, 2005. Production of rhamnolipids by Pseudomonas aeruginosa [J]. Applied Microbiology and Biotechnology, 68(6): 718-725.
DOI URL |
| [68] |
SODAGARI M, JU L K, 2020. Addressing the critical challenge for rhamnolipid production: Discontinued synthesis in extended stationary phase[J]. Process Biochemistry, 91: 83-89.
DOI URL |
| [69] |
SONG D D, LIANG S K, YAN L L, et al., 2016. Solubilization of polycyclic aromatic hydrocarbons by single and binary mixed rhamnolipid-sophorolipid biosurfactants[J]. Journal of Environmental Quality, 45(4): 1405-1412.
DOI URL |
| [70] |
SOTIROVA A V, SPASOVA D I, GALABOVA D N, et al., 2008. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains[J]. Current Microbiology, 56(6): 639-644.
DOI URL |
| [71] | SOUZA E C, VESSONI-PENNA T C, DE SOUZA OLIVEIRA R P, 2014. Biosurfactant-enhanced hydrocarbon bioremediation: An overview[J]. International Biodeterioration & Biodegradation, 89: 88-94. |
| [72] |
SUN S L, WANG Y X, ZANG T T, et al., 2019. A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons[J]. Bioresource Technology, 281: 421-428.
DOI URL |
| [73] | SYDOW Z, LISIECKI P, STANINSKA-PIĘTA J, et al., 2018. Multidimensional toxicity of rhamnolipid extracts obtained from creosote-contaminated soil[J]. CLEAN-Soil, Air, Water, 46(5): 1800053. 1-1800053.10. |
| [74] |
VARJANI S J, UPASANI V N, 2017. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant[J]. Bioresource Technology, 232: 389-397.
DOI URL |
| [75] |
WANG J F, BAO H Y, PAN G D, et al., 2021. Combined application of rhamnolipid and agricultural wastes enhances PAHs degradation via increasing their bioavailability and changing microbial community in contaminated soil[J]. Journal of Environmental Management, DOI: 10.1016/j.jenvman.2021.112998.
DOI |
| [76] |
WEI Y H, CHOU C L, CHANG J S, 2005. Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater[J]. Biochemical Engineering Journal, 27(2): 146-154.
DOI URL |
| [77] |
WITTGENS A, KOVACIC F, MULLER M M, et al., 2017. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors[J]. Applied Microbiology and Biotechnology, 101(7): 2865-2878.
DOI URL |
| [78] | WOLF D C, GAN J, 2018. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on (14)C-Pyrene mineralization in soil[J]. Environmental Pollution, 243(Pt B): 1846-1853. |
| [79] |
WU L M, LAI L, LU Q, et al., 2019. Comparative studies on the surface/interface properties and aggregation behavior of mono-rhamnolipid and di-rhamnolipid[J]. Colloids and Surfaces B: Biointerfaces, 181: 593-601.
DOI URL |
| [80] |
XU M, FU X, GAO Y, et al., 2020. Characterization of a biosurfactant-producing bacteria isolated from marine environment: Surface activity, chemical characterization and biodegradation[J]. Journal of Environmental Chemical Engineering, 8(5): 104277.
DOI URL |
| [81] |
ZANG T T, WU H Z, YAN B, et al., 2021. Enhancement of PAHs biodegradation in biosurfactant/phenol system by increasing the bioavailability of PAHs[J]. Chemosphere, DOI: 10.1016/j.chemosphere.2020.128941.
DOI |
| [82] |
ZENG Z T, LIU Y, ZHONG H, et al., 2018. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds[J]. Science of the Total Environment, 634: 1-11.
DOI URL |
| [83] | ZHANG D, ZHU L Z, 2014. Controlling microbiological interfacial behaviors of hydrophobic organic compounds by surfactants in biodegradation process[J]. Frontiers of Environmental Science & Engineering, 8(3): 305-315. |
| [84] |
ZHANG D, ZHU L Z, LI F, 2013. Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain[J]. Bioresource Technology, 142: 454-461.
DOI URL |
| [85] |
ZHANG L, VERES-SCHALNAT T A, SOMOGYI A, et al., 2012. Fatty acid cosubstrates provide beta-oxidation precursors for rhamnolipid biosynthesis in Pseudomonas aeruginosa, as evidenced by isotope tracing and gene expression assays[J]. Applied and Environmental Microbiology, 78(24): 8611-8622.
DOI URL |
| [86] |
ZHAO F, YUAN M L, LEI L Y, et al., 2021. Enhanced production of mono-rhamnolipid in Pseudomonas aeruginosa and application potential in agriculture and petroleum industry[J]. Bioresource Technology, DOI: 10.1016/j.biortech.2020.124605.
DOI |
| [87] |
ZHAO Z, SELVAM A, WONG J W, 2011. Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene[J]. Bioresource Technology, 102(5): 3999-4007.
DOI URL |
| [88] | ZHONG H, LIU G S, JIANG Y B, et al., 2017. Transport of bacteria in porous media and its enhancement by surfactants for bioaugmentation: A review[J]. Biotechnology Advances, 35(4): 490-504. |
| [89] |
ZHONG H, ZENG G M, YUAN X Z, et al., 2007. Adsorption of dirhamnolipid on four microorganisms and the effect on cell surface hydrophobicity[J]. Applied Microbiology and Biotechnology, 77(2): 447-455.
DOI URL |
| [90] | 陈来国, 冉勇 2004. 多环芳烃生物修复中的表面活性剂[J]. 生态环境学报, 13(1): 88-91. |
| CHEN L G, RAN Y, 2004. Surfactants in the bioremediation of PAHs[J]. Ecology and Environmental Sciences, 13(1): 88-91. | |
| [91] | 郭利果, 苏荣国, 梁生康, 等, 2009. 鼠李糖脂生物表面活性剂对多环芳烃的增溶作用[J]. 环境化学, 28(4): 510-514. |
| GUO L G, SU R G, LIANG S K, et al., 2009. Solubilization of polycyclic aromatic hydrocarbons by rhamnolipid biosurfactant[J]. Environmental Chemistry, 28(4): 510-514. | |
| [92] | 郎丽萍, 晏丽娟, 钟金魁, 等, 2015. 鼠李糖脂对多环芳烃的增溶作用及影响因素研究[J]. 安全与环境学报, 15(3): 267-270. |
| LANG L P, YAN L J, ZHONG J K, et al., 2015. Effect of rhamnolipid on the enhanced solubilization of the polycyclic aromatic hydrocarbons and the influential factors[J]. Journal of Safety and Environment, 15(3): 267-270. | |
| [93] | 刘有势, 马满英, 施周, 2012. 生物表面活性剂鼠李糖脂对PCBs污染土壤的修复作用研究[J]. 生态环境学报, 21(3): 559-563. |
| LIU Y S, MA M Y, SHI Z, 2012. Remediation of soil contaminated by polychlorinated biphenyls with rhamnolipid biosurfactant[J]. Ecology and Environmental Sciences, 21(3): 559-563. | |
| [94] | 马歌丽, 彭新榜, 马翠卿, 等, 2003. 生物表面活性剂及其应用[J]. 中国生物工程杂志, 23(5): 42-45. |
| MA G L, PENG X B, MA C Q, et al., 2003. The biosurfactants and its application[J]. China Biotechnology, 23(5): 42-45. | |
| [95] | 申国兰, 李利, 陈莎, 2018. 微生物降解石油源多环芳香烃的研究进展[J]. 土壤, 50(1): 16-27. |
| SHEN G L, LI L, CHEN S, 2018. Microbial degradation of polycyclic aromatic hydrocarbons from crude oils: a review[J]. Soils, 50(1): 16-27. | |
| [96] | 王晓旭, 孙丽娜, 郑学昊, 等, 2017. 表面活性剂强化微生物修复DDTs-PAHs复合污染农田土壤影响研究[J]. 生态环境学报, 26(3): 486-492. |
| WANG X X, SUN L N, ZHENG X H, et al., 2017. Enhanced effects of surfactants on the bioremediation of DDTs-PAHs in co-contaminated farmland soil[J]. Ecology and Environmental Sciences, 26(3): 486-492. | |
| [97] | 肖锟, 刘聪洋, 王仁女, 等, 2020. 表面活性剂影响多环芳烃微生物降解的研究进展[J]. 微生物学通报, 48(2): 582-595. |
| XIAO K, LIU C Y, WANG R N, et al., 2020. Effect of surfactants on microbial degradation of polycyclic aromatic hydrocarbons: a review[J]. Microbiology China, 48(2): 582-595. | |
| [98] | 肖鹏飞, 尤铁学, 宋玉珍, 等, 2014. 生物表面活性剂鼠李糖脂对白腐真菌降解DDTs的影响[J]. 环境科学与技术, 37(2): 29-33. |
| XIAO P F, YOU T X, SONG Y Z, et al., 2014. Effects of biosurfactant rhamnolipid on biodegradation of DDTs by white rot fungus[J]. Environmental Science and Technology, 37(2): 29-33. |
| [1] | 吉冰静, 刘艺, 吴杨, 高淑涛, 曾祥英, 于志强. 长江口及邻近东海沉积物中多环芳烃和含氧多环芳烃的分布特征、来源及生态风险[J]. 生态环境学报, 2022, 31(7): 1400-1408. |
| [2] | 程文远, 李法云, 吕建华, 吝美霞, 王玮. 碱改性向日葵秸秆生物炭对多环芳烃菲吸附特性研究[J]. 生态环境学报, 2022, 31(4): 824-834. |
| [3] | 张楷悦, 刘增辉, 王颜昊, 王敬宽, 崔德杰, 柳新伟. 黄河三角洲自然保护区土壤PAHs的风险评估和空间特征[J]. 生态环境学报, 2022, 31(11): 2198-2205. |
| [4] | 王飞, 赵颖. 太原市污灌区农田土壤中多环芳烃污染特征及生态风险评价[J]. 生态环境学报, 2022, 31(1): 160-169. |
| [5] | 蔡杨, 李伟, 左雪燕, 崔丽娟, 雷茵茹, 赵欣胜, 翟夏杰, 李晶, 潘旭. 盐城滨海湿地土壤多环芳烃分布特征及影响因素[J]. 生态环境学报, 2021, 30(6): 1249-1259. |
| [6] | 成庆利, 王大伟, 牛渤超, 张龙龙, 王雪娜, 张诗雨. 酶解预处理联合生物强化优化城市污泥好氧堆肥[J]. 生态环境学报, 2021, 30(12): 2395-2401. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
