生态环境学报 ›› 2023, Vol. 32 ›› Issue (12): 2216-2227.DOI: 10.16258/j.cnki.1674-5906.2023.12.013
李卓轩1( ), 彭自然1,2,*(
), 彭自然1,2,*( ), 何文辉1,2,3, 卫瑞璐1, 高琳茜1
), 何文辉1,2,3, 卫瑞璐1, 高琳茜1
收稿日期:2023-10-09
出版日期:2023-12-18
发布日期:2024-02-05
通讯作者:
*彭自然。E-mail: zrpeng@shou.edu.cn作者简介:李卓轩(1998年生),男,硕士研究生,研究方向为水污染控制及吸附材料。E-mail: zhuoxuan1998@163.com
基金资助:
LI Zhuoxuan1( ), PENG Ziran1,2,*(
), PENG Ziran1,2,*( ), HE Wenhui1,2,3, WEI Ruilu1, GAO Linxi1
), HE Wenhui1,2,3, WEI Ruilu1, GAO Linxi1
Received:2023-10-09
Online:2023-12-18
Published:2024-02-05
摘要:
为缓解水体富营养化,通过经济高效的方法降低水体氮磷含量,同时解决羊粪资源化利用问题,以羊粪球为原料在600 ℃下热解制备生物炭(YF600),利用Box-Behnken设计实验,研究浸渍比、pH、温度对YF600吸附水中NH4+-N、NO3−-N、PO43−-P效果的影响,运用响应面优化法确定最佳吸附条件。用吸附等温线中的Langmuir方程、Freundlich方程和吸附动力学的准一级方程、准二级方程对3种污染物的吸附过程进行拟合,并通过BET、FTIR、SEM-EDS、XRD、元素分析仪分析其理化性质和吸附机理。结果表明,对YF600吸附NH4+-N、PO43−-P的影响显著性大小为:浸渍比>pH>温度,吸附NO3−-N为:浸渍比>pH(温度影响不显著)。拟合出最佳吸附条件为浸渍比0.004,温度26.2 ℃,pH=6.77,此时NH4+-N、NO3−-N、PO43−-P的单位吸附量分别为14、2.08、4.93 mg∙g−1,实验结果与预测值基本吻合,说明YF600复合吸附氮磷效果较好,该模型具有可行性。Langmuir方程拟合更好证明其以単分子层吸附为主;准二级动力学模型能更好地反映YF600的吸附效率。表征结果为YF600比表面积达63.91 m2∙g−1,表面有大量褶皱且分布着不规则微孔,含丰富的羧基、酯基、羟基等含氧官能团和Mg、Ca、Fe、Al、Na、Si等多种元素,且Ca2+、Mg2+含量高,O/C高,以上理化性质使其具备良好的脱氮除磷性能,其氮磷吸附机理主要为离子交换、静电吸附和络合沉淀作用。研究结果表明,YF600复合吸附多种不同形式氮磷效果良好且工艺简单、生产成本低,对YF600氮磷吸附机理进行了解释分析,旨在为羊粪资源化利用与羊粪炭优化污水处理工艺提供理论依据。
中图分类号:
李卓轩, 彭自然, 何文辉, 卫瑞璐, 高琳茜. 羊粪炭对水体氮磷吸附条件的响应面优化及吸附机理研究[J]. 生态环境学报, 2023, 32(12): 2216-2227.
LI Zhuoxuan, PENG Ziran, HE Wenhui, WEI Ruilu, GAO Linxi. Response Surface Optimization and Adsorption Mechanism of Sheep Manure Charcoal on Nitrogen and Phosphorus Adsorption Conditions[J]. Ecology and Environment, 2023, 32(12): 2216-2227.
| 标号 | 因素 | 水平 |
|---|---|---|
| A | 浸渍比 | 0.001、0.003、0.005 |
| B | 吸附温度/℃ | 20、25、30 |
| C | pH | 5、7、9 |
表1 YF600吸附氮磷实验因素水平取值
Table 1 Level of experimental factors in the nitrogen and phosphorus
| 标号 | 因素 | 水平 |
|---|---|---|
| A | 浸渍比 | 0.001、0.003、0.005 |
| B | 吸附温度/℃ | 20、25、30 |
| C | pH | 5、7、9 |
| 序号 | 浸渍比 | 摄氏度/ ℃ | pH | NH4+-N 吸附量/ (mg∙g−1) | PO43−-P 吸附量/ (mg∙g−1) | NO3−-N 吸附量/ (mg∙g−1) |
|---|---|---|---|---|---|---|
| 1 | 0.001 | 20 | 7 | 8.14 | 1.66 | 0.89 |
| 2 | 0.005 | 20 | 7 | 11.38 | 4.26 | 2.02 |
| 3 | 0.001 | 30 | 7 | 7.66 | 2.52 | 0.91 |
| 4 | 0.005 | 30 | 7 | 12.38 | 4.52 | 2.08 |
| 5 | 0.001 | 25 | 5 | 6.23 | 2.35 | 1.17 |
| 6 | 0.005 | 25 | 5 | 8.22 | 3.80 | 2.37 |
| 7 | 0.001 | 25 | 9 | 6.38 | 2.84 | 0.83 |
| 8 | 0.005 | 25 | 9 | 10.27 | 4.92 | 1.79 |
| 9 | 0.003 | 20 | 5 | 8.77 | 3.68 | 1.98 |
| 10 | 0.003 | 30 | 5 | 11.17 | 3.95 | 2.01 |
| 11 | 0.003 | 20 | 9 | 11.25 | 4.73 | 1.35 |
| 12 | 0.003 | 30 | 9 | 11.01 | 4.90 | 1.32 |
| 13 | 0.003 | 25 | 7 | 14.11 | 4.84 | 1.88 |
| 14 | 0.003 | 25 | 7 | 13.94 | 4.55 | 1.77 |
| 15 | 0.003 | 25 | 7 | 13.86 | 4.66 | 1.87 |
| 16 | 0.003 | 25 | 7 | 14.39 | 4.88 | 1.75 |
| 17 | 0.003 | 25 | 7 | 13.88 | 4.93 | 1.88 |
表2 NH4+-N、NO3?-N、PO43?-P吸附实验设计及结果
Table 2 Adsorption experimental design and results of NH4+-N, NO3?-N and PO43?-P
| 序号 | 浸渍比 | 摄氏度/ ℃ | pH | NH4+-N 吸附量/ (mg∙g−1) | PO43−-P 吸附量/ (mg∙g−1) | NO3−-N 吸附量/ (mg∙g−1) |
|---|---|---|---|---|---|---|
| 1 | 0.001 | 20 | 7 | 8.14 | 1.66 | 0.89 |
| 2 | 0.005 | 20 | 7 | 11.38 | 4.26 | 2.02 |
| 3 | 0.001 | 30 | 7 | 7.66 | 2.52 | 0.91 |
| 4 | 0.005 | 30 | 7 | 12.38 | 4.52 | 2.08 |
| 5 | 0.001 | 25 | 5 | 6.23 | 2.35 | 1.17 |
| 6 | 0.005 | 25 | 5 | 8.22 | 3.80 | 2.37 |
| 7 | 0.001 | 25 | 9 | 6.38 | 2.84 | 0.83 |
| 8 | 0.005 | 25 | 9 | 10.27 | 4.92 | 1.79 |
| 9 | 0.003 | 20 | 5 | 8.77 | 3.68 | 1.98 |
| 10 | 0.003 | 30 | 5 | 11.17 | 3.95 | 2.01 |
| 11 | 0.003 | 20 | 9 | 11.25 | 4.73 | 1.35 |
| 12 | 0.003 | 30 | 9 | 11.01 | 4.90 | 1.32 |
| 13 | 0.003 | 25 | 7 | 14.11 | 4.84 | 1.88 |
| 14 | 0.003 | 25 | 7 | 13.94 | 4.55 | 1.77 |
| 15 | 0.003 | 25 | 7 | 13.86 | 4.66 | 1.87 |
| 16 | 0.003 | 25 | 7 | 14.39 | 4.88 | 1.75 |
| 17 | 0.003 | 25 | 7 | 13.88 | 4.93 | 1.88 |
| 参数 | NH4+-N吸附量/(mg∙g−1) | PO43−-P吸附量/(mg∙g−1) | NO3−-N吸附量/(mg∙g−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 平方和 | 自由度 | F | P | 显著性 | 平方和 | 自由度 | F | P | 显著性 | 平方和 | 自由度 | F | P | 显著性 | |||
| 模型 | 123.12 | 9 | 88.78 | <1×10−4 | 极显著 | 17.1 | 9 | 40.60 | <1×10−4 | 极显著 | 4.15 | 9 | 65.01 | <1×10−4 | 极显著 | ||
| A | 23.95 | 1 | 155.43 | <1×10−4 | 极显著 | 8.24 | 1 | 176.14 | <1×10−4 | 极显著 | 2.12 | 1 | 419.04 | <1×10−4 | 极显著 | ||
| B | 0.9 | 1 | 5.83 | 0.0465 | 显著 | 0.3 | 1 | 6.49 | 0.0383 | 显著 | 0.0046 | 1 | 0.16 | 0.7024 | |||
| C | 2.57 | 1 | 16.70 | 0.0047 | 极显著 | 1.61 | 1 | 34.39 | 6×10−4 | 极显著 | 0.011 | 1 | 107.84 | <1×10−4 | 显著 | ||
| AB | 0.54 | 1 | 3.53 | 0.1022 | 0.089 | 1 | 1.91 | 0.2098 | 0.004 | 1 | 0.078 | 0.7884 | |||||
| AC | 0.89 | 1 | 5.78 | 0.0472 | 显著 | 0.1 | 1 | 2.16 | 0.1855 | 2.25×10−8 | 1 | 2.32 | 0.1719 | ||||
| BC | 1.74 | 1 | 11.31 | 0.012 | 显著 | 0.0028 | 1 | 0.059 | 0.8152 | 0.0012 | 1 | 0.15 | 0.7084 | ||||
| A2 | 50.4 | 1 | 327.05 | <1×10−4 | 极显著 | 5.89 | 1 | 125.78 | <1×10−4 | 极显著 | 1.32 | 1 | 40.79 | <4×10−4 | 极显著 | ||
| B2 | 1.98 | 1 | 12.83 | 0.0089 | 显著 | 0.5 | 1 | 10.72 | 0.0136 | 显著 | 0.01 | 1 | 9.13 | 0.0193 | 显著 | ||
| C2 | 33.06 | 1 | 214.56 | <1×10−4 | 极显著 | 0.054 | 1 | 1.15 | 0.3199 | 0.55 | 1 | 1.73 | <0.2296 | 不显著 | |||
| 残差 | 1.08 | 7 | 0.33 | 7 | 0.013 | 7 | |||||||||||
| 失拟项 | 0.88 | 3 | 5.89 | 0.0598 | 不显著 | 0.22 | 3 | 2.82 | 0.1714 | 不显著 | 0.0079 | 3 | 1.97 | 0.2605 | 不显著 | ||
| 纯误差 | 0.2 | 4 | 0.11 | 4 | 0.0049 | 4 | |||||||||||
| 总误差 | 124.2 | 16 | 17.43 | 16 | 4.16 | 16 | |||||||||||
表3 NH4+-N、NO3?-N、PO43?-P单位吸附量方差分析结果
Table 3 Analysis of variance of unit sorption of NH4+-N, NO3?-N, PO43?-P by YF600
| 参数 | NH4+-N吸附量/(mg∙g−1) | PO43−-P吸附量/(mg∙g−1) | NO3−-N吸附量/(mg∙g−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 平方和 | 自由度 | F | P | 显著性 | 平方和 | 自由度 | F | P | 显著性 | 平方和 | 自由度 | F | P | 显著性 | |||
| 模型 | 123.12 | 9 | 88.78 | <1×10−4 | 极显著 | 17.1 | 9 | 40.60 | <1×10−4 | 极显著 | 4.15 | 9 | 65.01 | <1×10−4 | 极显著 | ||
| A | 23.95 | 1 | 155.43 | <1×10−4 | 极显著 | 8.24 | 1 | 176.14 | <1×10−4 | 极显著 | 2.12 | 1 | 419.04 | <1×10−4 | 极显著 | ||
| B | 0.9 | 1 | 5.83 | 0.0465 | 显著 | 0.3 | 1 | 6.49 | 0.0383 | 显著 | 0.0046 | 1 | 0.16 | 0.7024 | |||
| C | 2.57 | 1 | 16.70 | 0.0047 | 极显著 | 1.61 | 1 | 34.39 | 6×10−4 | 极显著 | 0.011 | 1 | 107.84 | <1×10−4 | 显著 | ||
| AB | 0.54 | 1 | 3.53 | 0.1022 | 0.089 | 1 | 1.91 | 0.2098 | 0.004 | 1 | 0.078 | 0.7884 | |||||
| AC | 0.89 | 1 | 5.78 | 0.0472 | 显著 | 0.1 | 1 | 2.16 | 0.1855 | 2.25×10−8 | 1 | 2.32 | 0.1719 | ||||
| BC | 1.74 | 1 | 11.31 | 0.012 | 显著 | 0.0028 | 1 | 0.059 | 0.8152 | 0.0012 | 1 | 0.15 | 0.7084 | ||||
| A2 | 50.4 | 1 | 327.05 | <1×10−4 | 极显著 | 5.89 | 1 | 125.78 | <1×10−4 | 极显著 | 1.32 | 1 | 40.79 | <4×10−4 | 极显著 | ||
| B2 | 1.98 | 1 | 12.83 | 0.0089 | 显著 | 0.5 | 1 | 10.72 | 0.0136 | 显著 | 0.01 | 1 | 9.13 | 0.0193 | 显著 | ||
| C2 | 33.06 | 1 | 214.56 | <1×10−4 | 极显著 | 0.054 | 1 | 1.15 | 0.3199 | 0.55 | 1 | 1.73 | <0.2296 | 不显著 | |||
| 残差 | 1.08 | 7 | 0.33 | 7 | 0.013 | 7 | |||||||||||
| 失拟项 | 0.88 | 3 | 5.89 | 0.0598 | 不显著 | 0.22 | 3 | 2.82 | 0.1714 | 不显著 | 0.0079 | 3 | 1.97 | 0.2605 | 不显著 | ||
| 纯误差 | 0.2 | 4 | 0.11 | 4 | 0.0049 | 4 | |||||||||||
| 总误差 | 124.2 | 16 | 17.43 | 16 | 4.16 | 16 | |||||||||||
| 污染物 | CV/% | r2 | r2Adj | r2Pred | 精密度 |
|---|---|---|---|---|---|
| Y1 | 3.65 | 0.9913 | 0.9801 | 0.8842 | 26.862 |
| Y2 | 5.41 | 0.9812 | 0.957 | 0.7865 | 19.371 |
| Y3 | 4.68 | 0.9882 | 0.973 | 0.8797 | 28.447 |
表4 可信度分析
Table 4 Reliability analysis
| 污染物 | CV/% | r2 | r2Adj | r2Pred | 精密度 |
|---|---|---|---|---|---|
| Y1 | 3.65 | 0.9913 | 0.9801 | 0.8842 | 26.862 |
| Y2 | 5.41 | 0.9812 | 0.957 | 0.7865 | 19.371 |
| Y3 | 4.68 | 0.9882 | 0.973 | 0.8797 | 28.447 |
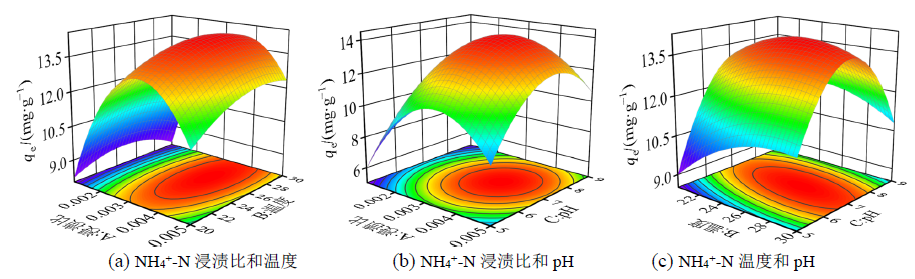
图1 浸渍比、温度和pH对NH4+-N吸附量交互影响响应面图
Figure 1 Response surface plots of the interaction effects of impregnation ratio, temperature and pH on NH4+-N adsorption
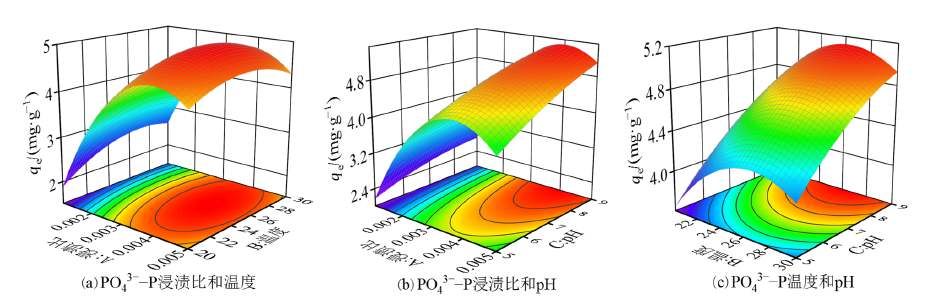
图2 浸渍比、温度和pH对PO43?-P吸附量交互影响响应面图
Figure 2 Response surface plots of the interaction effects of impregnation ratio, temperature and pH on PO43?-P adsorption
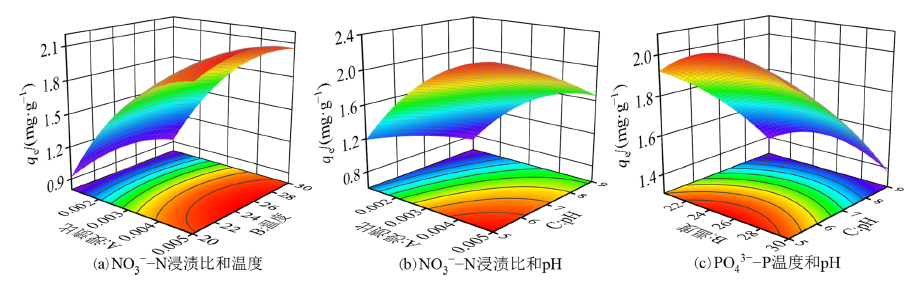
图3 浸渍比、温度和pH对NO3?-N吸附量交互影响响应面图
Figure 3 Response surface plots of the interaction effects of impregnation ratio, temperature and pH on NO3?-N adsorption
| 参数 | qe(NH4+-N)/(mg∙g−1) | qe(PO43−-N)/(mg∙g−1) | qe(NO3−-N)/(mg∙g−1) |
|---|---|---|---|
| 理论值 | 14.01 | 4.93 | 2.08 |
| 实际值1 | 14.00 | 4.91 | 1.98 |
| 实际值2 | 13.86 | 4.88 | 2.02 |
| 实际值3 | 13.98 | 4.88 | 2.01 |
| 平均值 | 13.95 | 4.89 | 2.00 |
| 偏差/% | 0.4±0.64 | 0.77±0.28 | 3.5±1.1 |
表5 YF600优化吸附条件实验结果
Table 5 Experimental results of optimized adsorption conditions of YF600
| 参数 | qe(NH4+-N)/(mg∙g−1) | qe(PO43−-N)/(mg∙g−1) | qe(NO3−-N)/(mg∙g−1) |
|---|---|---|---|
| 理论值 | 14.01 | 4.93 | 2.08 |
| 实际值1 | 14.00 | 4.91 | 1.98 |
| 实际值2 | 13.86 | 4.88 | 2.02 |
| 实际值3 | 13.98 | 4.88 | 2.01 |
| 平均值 | 13.95 | 4.89 | 2.00 |
| 偏差/% | 0.4±0.64 | 0.77±0.28 | 3.5±1.1 |
| 污染物 类型 | Langmuir方程 | Freundlich方程 | |||||
|---|---|---|---|---|---|---|---|
| qm /(mg∙g−1) | KL/(L∙g−1) | r2 | KF | 1/n | r2 | ||
| NH4+-N | 17.67 | 0.0116 | 0.9971 | 0.5215 | 0.6362 | 0.9931 | |
| NO3−-N | 2.46 | 0.1024 | 0.9706 | 0.9173 | 0.1997 | 0.937 | |
| PO43−-P | 4.89 | 0.1621 | 0.9946 | 1.3657 | 0.3293 | 0.9644 | |
表6 YF600对氮磷的等温吸附模型拟合参数
Table 6 Fitted parameters of isothermal adsorption model of YF600 on nitrogen and phosphorus
| 污染物 类型 | Langmuir方程 | Freundlich方程 | |||||
|---|---|---|---|---|---|---|---|
| qm /(mg∙g−1) | KL/(L∙g−1) | r2 | KF | 1/n | r2 | ||
| NH4+-N | 17.67 | 0.0116 | 0.9971 | 0.5215 | 0.6362 | 0.9931 | |
| NO3−-N | 2.46 | 0.1024 | 0.9706 | 0.9173 | 0.1997 | 0.937 | |
| PO43−-P | 4.89 | 0.1621 | 0.9946 | 1.3657 | 0.3293 | 0.9644 | |
| 污染物 | 拟一级动力学 | 拟二级动力学 | |||||
|---|---|---|---|---|---|---|---|
| qe/(mg∙g−1) | k1 | r2 | qe/(mg∙g−1) | k2 | r2 | ||
| NH4+-N | 5.56 | 0.4776 | 0.9695 | 9.59 | 0.2583 | 0.9977 | |
| NO3−-N | 1.29 | 0.3240 | 0.977 | 2.57 | 0.934 | 0.9972 | |
| PO43−-P | 4.65 | 0.4653 | 0.9824 | 4.59 | 0.4266 | 0.9966 | |
表7 YF600的吸附动力学拟合参数
Table 7 Adsorption kinetics fitting parameters of YF600
| 污染物 | 拟一级动力学 | 拟二级动力学 | |||||
|---|---|---|---|---|---|---|---|
| qe/(mg∙g−1) | k1 | r2 | qe/(mg∙g−1) | k2 | r2 | ||
| NH4+-N | 5.56 | 0.4776 | 0.9695 | 9.59 | 0.2583 | 0.9977 | |
| NO3−-N | 1.29 | 0.3240 | 0.977 | 2.57 | 0.934 | 0.9972 | |
| PO43−-P | 4.65 | 0.4653 | 0.9824 | 4.59 | 0.4266 | 0.9966 | |
| 样品 | 产率/% | w(N)/% | w(C)/% | w(O)/% | w(P)/% | w(H)/% | w(Mg)/% | H/C | 比表面积/(m2∙g−1) | 平均孔径/nm |
|---|---|---|---|---|---|---|---|---|---|---|
| YF600 | 33.36 | 1.43 | 54.23 | 28.56 | 3.51 | 2.01 | 1.44 | 0.037 | 63.91 | 2.79 |
表8 生物炭的理化特征
Table 8 Physicochemical characteristics of each biochar
| 样品 | 产率/% | w(N)/% | w(C)/% | w(O)/% | w(P)/% | w(H)/% | w(Mg)/% | H/C | 比表面积/(m2∙g−1) | 平均孔径/nm |
|---|---|---|---|---|---|---|---|---|---|---|
| YF600 | 33.36 | 1.43 | 54.23 | 28.56 | 3.51 | 2.01 | 1.44 | 0.037 | 63.91 | 2.79 |
| [1] |
ALMAHBASHI N M Y, KUTTY S R M, AYOUB M, et al., 2021. Optimization of preparation conditions of sewage sludge based activated carbon[J]. Ain Shams Engineering Journal, 12(2): 1175-1182.
DOI URL |
| [2] |
ANGAR, YASSMIN, DJELALI, et al., 2017. Investigation of ammonium adsorption on Algerian natural bentonite[J]. Environmental Science and Pollution Research, 24(12): 11078-11089.
DOI URL |
| [3] |
AREF A, HE H P, ZHU J X, et al., 2018. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms[J]. Applied Clay Science, 159: 83-93.
DOI URL |
| [4] |
CHENG N, WANG B, FENG Q W, et al., 2021. Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water[J]. Bioresource Technology, 340: 125696.
DOI URL |
| [5] |
CUI X Q, HAO H L, HE Z L, et al., 2016. Pyrolysis of wetland biomass waste: Potential for carbon sequestration and water remediation[J]. Journal of Environmental Management, 173: 95-104.
DOI PMID |
| [6] |
DANIEL J, CONLEY, HANS W, et al., 2009. Controlling eutrophication: Nitrogenand phosphorus[J]. Science, 323(5917): 1014-1015.
DOI URL |
| [7] |
DILEKOGLU M F, YAPICI M, 2023. Adsorption of naproxen pharmaceutical micropollutant from aqueous solutions on superior activated carbon synthesized from sheep manure: Kinetics, thermodynamics, and mechanism[J]. Journal of Molecular Liquids, 381: 121839.
DOI URL |
| [8] |
EUFRASIO P, MARINA D C, DA S, et al., 2019. Biochar from carrot residues chemically modified with magnesium for removing phosphorus from aqueous solution[J]. Journal of Cleaner Production, 222: 36-46.
DOI URL |
| [9] |
GAI X P, WANG H Y, LIU J, et al., 2014. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate[J]. PLoS One, 9(12): e113888.
DOI URL |
| [10] |
GELARDI D L, LI C Y, PARIKH S J, et al., 2019. An emerging environmental concern: Biochar-induced dust emissions and their potentially toxic properties[J]. Science of The Total Environment, 678: 813-820.
DOI URL |
| [11] |
HASSAN M, LIU Y J, NAIDU R, et al., 2020. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis[J]. Science of The Total Environment, 744: 140714.
DOI URL |
| [12] |
JENSEN W A, 2017. Response surface methodology: Process and product optimization using designed experiments 4th edition[J]. Journal of Quality Technology, 49(2): 186-187.
DOI URL |
| [13] |
JUNG K W, HWANG M J, AHN K H, 2015. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media[J]. International Journal of Environmental Science and Technology, 12(10): 3363-3372
DOI URL |
| [14] |
KHAIRUDDIN M I, SUHARDY D, NASRUL H, et al., 2011. Thermogravimetric analysis and the optimisation of bio-oil yield from fixed-bed pyrolysis of rice husk using response surface methodology (RSM)[J]. Industrial Crops and Products, 33(2): 481-487.
DOI URL |
| [15] |
KIZITO S, WU S B, KIRUI W K, et al., 2015. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry[J]. Science of The Total Environment, 505: 102-112.
DOI URL |
| [16] |
LI H Q, HU J T, MENG Y, et al., 2017. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather[J]. Science of The Total Environment, 603-604: 39-48.
DOI URL |
| [17] |
LI Y, LIU Y, WANG H Y, et al., 2023. In situ remediation mechanism of internal nitrogen and phosphorus regeneration and release in shallow eutrophic lakes by combining multiple remediation techniques[J]. Water Research, 229: 119394.
DOI URL |
| [18] |
LIAO P, YUAN S H, XIE W J, et al., 2013. Adsorption of nitrogen-heterocyclic compounds on bamboo charcoal: Kinetics, thermodynamics, and microwave regeneration[J]. Journal of Colloid and Interface Science, 390(1): 189-195.
DOI PMID |
| [19] |
MAHTAB A, ANUSHKA U R, JUNG E L, et al., 2014. Biochar as a sorbent for contaminant management in soil and water: A review[J]. Chemosphere, 99: 19-33.
DOI PMID |
| [20] |
NAUTIYAL, PIYUSHI, SUBRAMANIAN, et al., 2016. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry[J]. Journal of Environmental Management, 182: 187-197.
DOI PMID |
| [21] |
NOVAIS S V, ZENERO M D O, BARRETO M S C, et al., 2018. Phosphorus removal from eutrophic water using modified biochar[J]. Science of The Total Environment, 633: 825-835.
DOI URL |
| [22] |
QIN B Q, ZHANG Y L, ZHU G W, et al., 2023. Eutrophication control of large shallow lakes in China[J]. Science of The Total Environment, 881: 163494.
DOI URL |
| [23] |
QU M J, LI N, LI H D, et al., 2018. Phytoextraction and biodegradation of atrazine by Myriophyllum spicatum and evaluation of bacterial communities involved in atrazine degradation in lake sediment[J]. Chemosphere, 209: 439-448.
DOI PMID |
| [24] |
SHEN Z F, LIU C L, YIN C C, et al., 2019. Facile large-scale synthesis of macroscopic 3D porous graphene-like carbon nanosheets architecture for efficient CO2 adsorption[J]. Carbon, 145(5867): 751-756.
DOI URL |
| [25] | TAKAYA C A, FLETCHER L A, SINGH S, et al., 2016. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes[J]. Chemosphere: Environmental Toxicology and Risk Assessment, 145: 518-527. |
| [26] |
WAN S, WANG S S, LI Y C, et al., 2017. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions[J]. Journal of Industrial and Engineering Chemistry, 47: 246-253.
DOI URL |
| [27] | WANG B, LIAN G Q, LEE X Q, et al., 2020. Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water[J]. Chemosphere: Environmental Toxicology and Risk Assessment, 238: 124684. 1-124684.8. |
| [28] | WANG Z F, WANG H, LI Q M, et al., 2016. pH effect on Re(VII) and Se(IV) diffusion in compacted GMZ bentonite[J]. Applied Geochemistry: Journal of the International Association of Geochemistry and Cosmochemistry, 73: 1-7. |
| [29] |
WANG Z Y, BAKSHI S, LI C Y, et al., 2020. Modification of pyrogenic carbons for phosphate sorption through binding of a cationic polymer[J]. Journal of Colloid and Interface Science, 579: 258-268.
DOI PMID |
| [30] |
YANG H I, LOU K, RJAPAKSHA A U, et al., 2018. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars[J]. Environmental Science and Pollution Research, 25(26): 25638-25647.
DOI |
| [31] |
YE Y Y, NGO H H, GUO, W S, et al., 2017. Insight into chemical phosphate recovery from municipal wastewater[J]. Science of the Total Environment, 576: 159-171.
DOI URL |
| [32] | ZENG Z, ZHANG S D, LI T Q, et al., 2013. Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants[J]. Journal of Zhejiang University (Engineering Science), 14(12): 1152-1161. |
| [33] |
ZHANG L F, HUANG X D, FU G K, et al., 2023. Aerobic electrotrophic denitrification coupled with biologically induced phosphate precipitation for nitrogen and phosphorus removal from high-salinity wastewater: Performance, mechanism, and microbial community[J]. Bioresource Technology, 372: 128696.
DOI URL |
| [34] | ZHANG M, SONG G, GELARDI D L, et al., 2020. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water[J]. Water research: A journal of the international water association, 186: 116303. |
| [35] |
ZHOU L, XU D F, Ll Y X, et al., 2019. Phosphorus and nitrogen adsorption capacities of biochars derived from feedstocksat different pyrolysis temperatures[J]. Water, 11(8): 1559.
DOI URL |
| [36] | ZHUANG X Z, GAN Z Y, CEN K H, et al., 2022. Upgrading biochar by co-pyrolysis of heavy bio-oil and apricot shell using response surface methodology[J]. Fuel, 310(Part C): 122447. |
| [37] | 陈刚, 朱赫特, 陈浩然, 等, 2023. 镁改性水生植物生物炭吸附水中的微囊藻毒素-LR[J]. 环境化学, 1-14 [2024-01-04] http://kns.cnki.net/kcms/detail/11.1844.X.20230424.2120.024.html. |
| [38] | CHEN G, ZHU H T, CHEN H R, et al., 2023. Adsorption of microcystin-LR by Mg- modified aquatic plant biochar in water[J]. Environmental Chemistry, 1-14[2024-01-04]http://kns.cnki.net/kcms/detail/11.1844.X.20230424.2120.024.html. |
| [39] | 陈梅, 王芳, 张德俐, 等, 2019. 生物炭结构性质对氨氮的吸附特性影响[J]. 环境科学, 40(12): 5421-5429. |
| CHEN M, WANG F, ZHANG D L, et al., 2019. Effect of biochar structure on adsorption characteristics of ammonia nitrogen[J]. Environmental Science, 40(12): 5421-5429. | |
| [40] | 陈明茹, 黄应平, 张吉林, 等, 2022. 羊粪生物炭对水体中镉的吸附[J]. 武汉大学学报(理学版), 68(6): 612-620. |
| CHEN M R, HUANG Y P, ZHANG J L, et al., 2022. Adsorption of cadmium in water by sheep manure biochar[J]. Journal of Wuhan University (Natural Science Edition), 68(6): 612-620. | |
| [41] |
程文远, 李法云, 吕建华, 等, 2022. 碱改性向日葵秸秆生物炭对多环芳烃菲吸附特性研究[J]. 生态环境学报, 31(4): 824-834.
DOI |
| CHENG W Y, LI F Y, LÜ J H, et al., 2022. Sorption Characteristics of polycyclic aromatic hydrocarbons phenanthrene on sunflower straw biochar modified with alkali[J]. Ecology and Environment, 31(4): 824-834. | |
| [42] | 冯弋洋, 罗元, 何秋平, 等, 2020. La改性羊粪生物炭吸附水体磷酸盐特性研究[J]. 农业环境科学学报, 39(10): 2380-2386. |
| FENG G Y, LUO Y, HE Q P, et al., 2020. Adsorption of phosphate from water by La-modified sheep manure biochar[J]. Journal of Agro-Environment Science, 39(10): 2380-2386. | |
| [43] | 何雨, 罗云霞, 樊仙, 等, 2023. 淹水条件下羊粪炭对重金属复合污染土壤中Pb Zn有效性影响[J]. 广东农业科学, 1-11 [2024-01-04] http://kns.cnki.net/kcms/detail/44.1267.s.20230922.1028.004.html. |
| HE Y, LUO Y X, FAN X, et al., 2023. Effect of biochar application under flooded conditions on the availability of Pb and Zn in heavy metal contaminated soil[J]. Guangdong Agricultural Sciences, 1-11 [2024-01-04] http://kns.cnki.net/kcms/detail/44.1267.s.20230922.1028.004.html. | |
| [44] | 胡锦刚, 肖春桥, 邓祥意, 等, 2022. 稀土矿山氨氮废水生物脱氮方法研究进展[J]. 武汉工程大学学报, 44(1): 1-8. |
| HU J G, XIAO C Q, DENG X Y, et al., 2022. Research progress in biological denitrification methods of ammonia nitrogen wastewater from rare earth mines[J]. Journal of Wuhan Institute of Technology, 44(1): 1-8. | |
| [45] | 黄俊亮, 刘成, 邱超, 等, 2021. 离子交换生物脱氮组合工艺去除饮用水中硝酸盐[J]. 环境工程学报, 15(6): 1894-1904. |
| HUANG J L, LIU C, QIU C, et al., 2021. Removal of nitrate from drinking water by combined process of ion exchangeand biological denitrification[J]. Chinese Journal of Environmental Engineering, 15(6): 1894-1904. | |
| [46] | 黄雯, 张雪萍, 张建强, 等, 2022. 磁改性羊粪衍生ZVI-生物炭的制备及其活化过一硫酸盐降解AO7特性研究[J]. 环境科学学报, 42(7): 196-208. |
| HUANG W, ZHANG X P, ZHANG J Q, et al., 2022. Preparation of ZVI-biochar derived from magnetically modified sheep manure and its activation of peroxymonosulfate to degrade AO7[J]. Acta Scientiae Circumstantiae, 42(7): 196-208. | |
| [47] | 李娜, 黎佳茜, 李国文, 等, 2018. 中国典型湖泊富营养化现状与区域性差异分析[J]. 水生生物学报, 42(4): 854-864. |
| LI N, LI J X, LI G W, et al., 2018. The eutrophication its regional heterogeneity in typical lakes of China[J]. Acta Hydrobiologica Sinica, 42(4): 854-864. | |
| [48] | 李书田, 刘荣乐, 陕红, 2009. 我国主要畜禽粪便养分含量及变化分析[J]. 农业环境科学学报, 28(1): 179-184. |
| LI S T, LIU R L, SHAN H, 2009. Nutrient contents in main animal manures in China[J]. Journal of Agro-environment Science, 28(1): 179-184. | |
| [49] | 梁嘉琪, 吕媛, 陆茵, 等, 2020. 铁磁性氧化镁生物炭对玉米加工废水中氮磷的回收效果[J]. 环境工程, 38(9): 89-94. |
| LIANG J Q, LÜ Y, LU Y, et al., 2020. Recovery of ammonium and phosphate from corn processing wastewater using magnetic MgO-biochar[J]. Environmental Engineering, 38(9): 89-94. | |
| [50] | 马路路, 许利滢, 张泽新, 等, 2023. 废弃香蕉皮粉末对六价铬的吸附特性与机理研究[J]. 生态与农村环境学报, 1-17 [2024-01-04]. https://doi.org/10.19741/j.issn.1673-4831.2023.0816. |
| MA L L, XU L Y, ZHANG Z X, et al., 2023. Study on adsorption characteristics and mechanism of Cr(VI) by waste banana peel powder[J]. Journalof Ecology and Rural Environment, 1-17 [2024-01-04]. https://doi.org/10.19741/j.issn.1673-4831.2023.0816. | |
| [51] | 秦帆, 王玥, 黄亚楠, 等, 2018. 改性秸秆生物质炭吸附去除水中氨氮的研究[J]. 森林工程, 34(3): 19-25, 31. |
| QIN F, WANG Y, HUANG Y N, et al., 2018. Study on adsorption removal of ammonia nitrogen in water by modified straw biochar[J]. Forest Engineering, 34(3): 19-25, 31. | |
| [52] |
唐鑫磊, 邢涛, 夏金雨, 等, 2023. 镁改性生物炭吸附水和畜禽养殖废水中氮磷的研究[J]. 工业水处理, 43(9): 144-152.
DOI |
|
TANG X L, XING T, XIA J Y, et al., 2023. Adsorption of nitrogen and phosphorus in water and livestock and poultry wastewater by magnesium-modified biochar[J]. Industrial Water Treatment, 43(9): 144-152.
DOI |
|
| [53] | 汪淑廉, 王永昌, 张宇, 等, 2022. 改性花生壳生物炭对磷酸盐的吸附特性[J]. 环境科学与技术, 45(S1): 21-26. |
|
WANG S L, WANG Y C, ZHANG Y, et al., 2022. Adsorption properties of phosphorus by modified peanut shell biochar[J]. Environmental Science & Technology, 45(S1): 21-26.
DOI URL |
|
| [54] | 王硕, 汪雅茹, 尹通, 等, 2023. 改性生物炭对水体中氮和磷共去除: 改性方法和吸附机制[J]. 化学试剂, 45(7): 119-127 WANG S, WANG Y R, YIN T, et al. |
| 2023. Co-removal of nitrogen and phosphate from water by modified biochar: modification methods and adsorption mechanisms[J]. Chemical Reagents, 45(7): 119-127. | |
| [55] | 王怡, 陈琳风, 王文怀, 等, 2019. 改性石榴皮生物炭对水中低浓度硝氮的吸附性能研究[J]. 西安建筑科技大学学报(自然科学版), 51(6): 899-904. |
| WANG Y, CHEN L F, WANG W H, et al., 2019. Adsorption behavior of low concentration nitrate nitrogen from water by modified biochars from pomegranate peel[J]. Journal of Xi’an University of Architecture & Technology, 51(6): 899-904. | |
| [56] | 吴奇, 谭美涛, 迟道才, 2022. 生物炭吸附富营养化水体氮、磷的研究进展[J]. 沈阳农业大学学报, 53(5): 620-629. |
| WU Q, TAN M T, CHI D C, 2022. Research progress of biochar on adsorption of nitrogen and phosphorus in eutrophic water[J]. Journal of Shenyang Agricultural University, 53(5): 620-629. | |
| [57] | 向江涛, 黄应平, 凌海波, 等, 2019. 羊粪生物炭对水体氨氮吸附特性研究[J]. 环境科学与技术, 42(7): 147-153. |
|
XIANG J T, HUANG Y P, LING H B, et al., 2019. Adsorption characteristics of ammonia nitrogen from water in sheep manure biochar[J]. Environmental Science & Technology, 42(7): 147-153.
DOI URL |
|
| [58] | 谢淘, 2015. 生物炭的特性分析及其在黄水资源化中的应用[D]. 北京: 清华大学. |
| XIE T, 2015. Characterization of biochar and its application in yellow water treatment[D]. Beijing: Tsinghua University. | |
| [59] | 张超, 翟付杰, 单保庆, 2023. Ca改性生物炭对土壤磷赋存形态影响及稳定化机制[J]. 环境科学, 1-17 [2024-01-04] https://doi.org/10.13227/j.hjkx.202211160. |
| ZHANG C, ZHAI F J, SHAN B Q, 2023. Effect of Ca Modified Biochar on the Chemical Speciation of Soil[J]. Environmental Science, 1-17. [2024-01-04] https://doi.org/10.13227/j.hjkx.202211160. | |
| [60] | 张璐, 贾丽, 陆文龙, 等, 2015. 不同碳化温度下玉米秸秆生物炭的结构性质及其对氮磷的吸附特性[J]. 吉林大学学报 (理学版), 53(4): 802-808. |
| ZHANG L, JIA L, LU W L, et al., 2015. Structural properties of corn straw biochar and characteristics of its adsorption for nitrogen and phosphate at different carbonization temperature[J]. Journal of Jilin University (Science Edition), 53(4): 802-808. | |
| [61] |
张太平, 肖嘉慧, 胡凤洁, 2021. 生物炭固定化微生物技术在去除水中污染物的应用研究进展[J]. 生态环境学报, 30(5): 1084-1093.
DOI |
| ZHANG T P, XIAO J H, HU F J, 2021. Research progress in the removal of contaminants from water by immobilized microorganisms combined with biochar[J]. Ecology and Environment, 30(5): 1084-1093. | |
| [62] | 张文, 吕欣田, 韩睿, 等, 2018. 2种改性生物炭对水体硝态氮的吸附特性[J]. 生态与农村环境学报, 34(3): 253-259. |
| ZHANG W, LÜ X T, HAN R, et al., 2018. Effects of two kinds of modified biochar adsorbing nitrate-n in Water[J]. Journal of Ecology and Rural Environment, 34(3): 253-259. |
| [1] | 赵维彬, 唐丽, 王松, 刘玲玲, 王树凤, 肖江, 陈光才. 两种生物炭对滨海盐碱土的改良效果[J]. 生态环境学报, 2023, 32(4): 678-686. |
| [2] | 苏丹, 罗桥冰, 董昱杉, 杨彩霞, 王鑫. 混合型生物炭对寒冷地区PAHs污染土壤微生物修复的强化作用[J]. 生态环境学报, 2023, 32(11): 1942-1951. |
| [3] | 赵丹丹, 李文健, 江丽霞, 单锐, 陈德珍, 袁浩然, 陈勇. 生物炭基光催化剂的制备及其降解废水中的有机污染物研究进展[J]. 生态环境学报, 2023, 32(11): 2019-2029. |
| [4] | 陈桂红. 硫和硅掺杂生物炭对镉污染土壤的修复研究[J]. 生态环境学报, 2023, 32(10): 1854-1860. |
| [5] | 游宏建, 张文文, 兰正芳, 马兰, 张宝娣, 穆晓坤, 李文慧, 曹云娥. 蚯蚓原位堆肥与生物炭对黄瓜根结线虫及根际微生物的影响[J]. 生态环境学报, 2023, 32(1): 99-109. |
| [6] | 张林, 周飘, 齐实, 张岱, 伍冰晨, 崔冉冉. 侧柏人工林林分空间结构对林下草本多样性的差异性影响及其关联度[J]. 生态环境学报, 2022, 31(9): 1794-1801. |
| [7] | 李晓晖, 艾仙斌, 李亮, 王玺洋, 辛在军, 孙小艳. 新型改性稻壳生物炭材料对镉污染土壤钝化效果的研究[J]. 生态环境学报, 2022, 31(9): 1901-1908. |
| [8] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| [9] | 房献宝, 张智钧, 赖阳晴, 叶脉, 刁增辉. 新型污泥生物炭对土壤重金属Cr和Cd的修复研究[J]. 生态环境学报, 2022, 31(8): 1647-1656. |
| [10] | 钱莲文, 余甜甜, 梁旭军, 王义祥, 陈永山. 茶园土壤酸化改良中生物炭应用5 a后的稳定性研究[J]. 生态环境学报, 2022, 31(7): 1442-1447. |
| [11] | 张慧琦, 李子忠, 秦艳. 玉米秸秆生物炭用量对砂土孔隙和持水性的影响[J]. 生态环境学报, 2022, 31(6): 1272-1277. |
| [12] | 邓晓, 武春媛, 杨桂生, 李怡, 李勤奋. 椰壳生物炭对海南滨海土壤的改良效果[J]. 生态环境学报, 2022, 31(4): 723-731. |
| [13] | 魏岚, 黄连喜, 李翔, 王泽煌, 陈伟盛, 黄庆, 黄玉芬, 刘忠珍. 生物炭基质可显著地促进香蕉幼苗生长[J]. 生态环境学报, 2022, 31(4): 732-739. |
| [14] | 赵超凡, 周丹丹, 孙建财, 钱坤鹏, 李芳芳. 生物炭中可溶性组分对其吸附镉的影响[J]. 生态环境学报, 2022, 31(4): 814-823. |
| [15] | 程文远, 李法云, 吕建华, 吝美霞, 王玮. 碱改性向日葵秸秆生物炭对多环芳烃菲吸附特性研究[J]. 生态环境学报, 2022, 31(4): 824-834. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
