生态环境学报 ›› 2022, Vol. 31 ›› Issue (1): 151-159.DOI: 10.16258/j.cnki.1674-5906.2022.01.017
收稿日期:2021-10-25
出版日期:2022-01-18
发布日期:2022-03-10
通讯作者:
*陶亮,男,研究员,博士,主要从事微观土壤界面化学过程及其环境效应等领域研究。E-mail: taoliang@soil.gd.cn作者简介:陈文洁(1998年生),女,硕士研究生,主要从事土壤界面化学过程研究。E-mail: 1614230745@qq.com
基金资助:
CHEN Wenjie1,2( ), LI Hui3, HE Bin2, TAO Liang2,4,*(
), LI Hui3, HE Bin2, TAO Liang2,4,*( )
)
Received:2021-10-25
Online:2022-01-18
Published:2022-03-10
摘要:
磷素(P)兼具重要养分元素的利和潜在面源污染的弊,其在土壤环境中的固存行为及其迁移转化过程受到广泛关注。该研究选取黄、红壤中典型矿物(针铁矿、赤铁矿及高岭石)为模式矿物,在排除pH干扰的条件下,开展了共存阴(As(V))阳(Cd(II))离子对矿物表面P(V)固存机制的影响研究。结果表明:P(V)在不同矿物表面的吸附效率表现为针铁矿>赤铁矿>高岭石;阴离子As(V)、阳离子Cd(II)与P(V)共存并不会改变P(V)在针铁矿表面的吸附动力学特征,仍符合准二级动力学模型,化学吸附为其控速步骤;P(V)与As(V)共存时,随着As(V)浓度的增加,P(V)的吸附等温线呈现从Langmuir型向Freundlich型转变的趋势,吸附量减小,但吸附速率增大,As(V)主要通过静电排斥作用和吸附位点竞争作用降低P(V)在针铁矿表面的吸附量;P(V)与Cd(II)共存时,P(V)的吸附速率先增加后减小。具体来说,Cd(II)/P(V)物质的量比值<0.5时,Cd(II)主要通过静电吸引略微促进P(V)在针铁矿表面的吸附;Cd(II)/P(V)物质的量比值>0.5时,静电吸附和形成Fe-P(V)-Cd(II)三元络合物是促进针铁矿表面P(V)固存的主要调控机制;进一步增加Cd(II)/P(V)物质的量比值,P(V)固存的主要调控机制逐步转变为形成P(V)与Cd(II)的表面共沉淀。该结果可为研究P在土壤环境中固存的微观机制及其关键影响因子提供基础研究数据,也有望为提高P的利用率以及为调控P的面源污染问题提供有益帮助。
中图分类号:
陈文洁, 李慧, 贺斌, 陶亮. 共存阴阳离子对针铁矿表面磷固存机制的影响研究[J]. 生态环境学报, 2022, 31(1): 151-159.
CHEN Wenjie, LI Hui, HE Bin, TAO Liang. Influence of Co-existing Anions and Cations on Phosphate Sequestration onto Goethite[J]. Ecology and Environment, 2022, 31(1): 151-159.
| 矿物 Minerals | Qe/(μmol∙g-1) | SSA/(m2∙g-1) | Qe’/(μmol∙m-2) |
|---|---|---|---|
| 针铁矿 Goethite | 232.04 | 81.15 | 2.86 |
| 赤铁矿 Hematite | 18.71 | 29.19 | 0.64 |
| 高岭石 Kaolinite | 2.78 | 15.70 | 0.18 |
表1 P(V)在不同矿物表面的归一化吸附效率(pH=6.0)(黄敏雪等,2022)
Table 1 The normalized adsorption efficiency of P (V) onto different mineral surfaces (pH=6.0)
| 矿物 Minerals | Qe/(μmol∙g-1) | SSA/(m2∙g-1) | Qe’/(μmol∙m-2) |
|---|---|---|---|
| 针铁矿 Goethite | 232.04 | 81.15 | 2.86 |
| 赤铁矿 Hematite | 18.71 | 29.19 | 0.64 |
| 高岭石 Kaolinite | 2.78 | 15.70 | 0.18 |
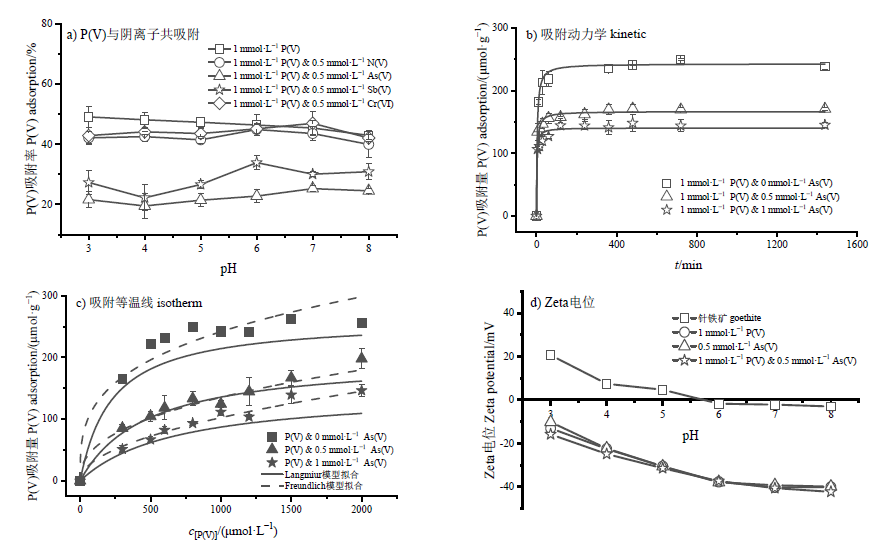
图3 不同阴离子对P(V)在针铁矿界面吸附的影响(a),P(V)与As(V)共吸附的P(V)吸附动力学(b)和吸附等温线(c),P(V)与As(V)共吸附的针铁矿表面Zeta电位变化情况(d)
Figure 3 Effects of anions on P(V) adsorption onto goethite (a), kinetics of P(V) adsorption (b), isotherms of P(V) adsorption when P(V) co-adsorbed with As(V) onto goethite (c), and Zeta potential values when P(V) co-adsorbed with As(V) onto goethite surface (d)
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ (min-1) | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 88.89 | 0.4954 | 0.835 | 166.67 | 0.0026 | 0.999 | |
| P(V)3 | 72.59 | 0.4290 | 0.701 | 140.65 | 0.0038 | 0.996 | |
表2 As(V)存在下的P(V)吸附动力学拟合参数
Table 2 Fitting parameter of P(V) adsorption kinetic when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ (min-1) | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 88.89 | 0.4954 | 0.835 | 166.67 | 0.0026 | 0.999 | |
| P(V)3 | 72.59 | 0.4290 | 0.701 | 140.65 | 0.0038 | 0.996 | |
| 吸附质 Adsorbate | Langmuir模型 Langmuir model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| Qm/ (μmol∙g-1) | KL/ (L∙μmol-1) | R2 | n | KF/(μmol∙g-1)∙ (μmol∙L-1)n | R2 | ||
| P(V)1 | 262.10 | 0.0045 | 0.988 | 3.29 | 29.82 | 0.900 | |
| P(V)2 | 197.97 | 0.0021 | 0.922 | 2.44 | 7.98 | 0.917 | |
| P(V)3 | 146.47 | 0.0015 | 0.895 | 1.99 | 3.19 | 0.904 | |
表3 As(V)存在下的P(V)吸附等温线拟合参数
Table 3 The fitting parameter of P(V) adsorption isotherm when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | Langmuir模型 Langmuir model | Freundlich模型 Freundlich model | |||||
|---|---|---|---|---|---|---|---|
| Qm/ (μmol∙g-1) | KL/ (L∙μmol-1) | R2 | n | KF/(μmol∙g-1)∙ (μmol∙L-1)n | R2 | ||
| P(V)1 | 262.10 | 0.0045 | 0.988 | 3.29 | 29.82 | 0.900 | |
| P(V)2 | 197.97 | 0.0021 | 0.922 | 2.44 | 7.98 | 0.917 | |
| P(V)3 | 146.47 | 0.0015 | 0.895 | 1.99 | 3.19 | 0.904 | |
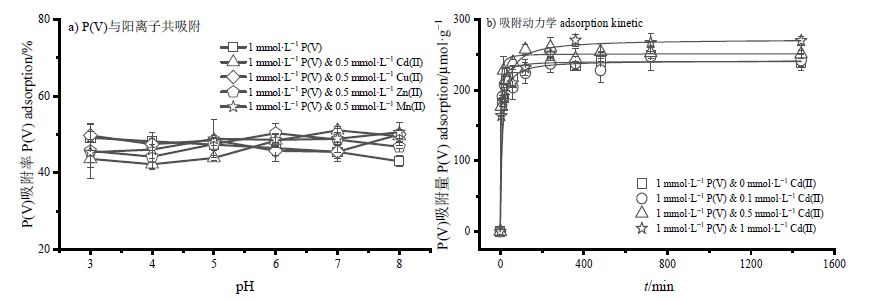
图4 不同阳离子对P(V)在针铁矿界面吸附的影响(a),P(V)与Cd(II)在针铁矿界面共吸附的P(V)吸附动力学(b)
Figure 4 Effects of cations on P(V) adsorption onto goethite (a), P(V) adsorption kinetic when P (V) co-adsorbed with Cd(II) onto goethite (b)
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ min-1 | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 130.69 | 0.3042 | 0.775 | 240.96 | 0.0010 | 0.999 | |
| P(V)3 | 402.49 | 0.5954 | 0.899 | 251.89 | 0.0041 | 0.999 | |
| P(V)4 | 174.82 | 0.6929 | 0.406 | 271.74 | 0.0003 | 0.999 | |
表4 Cd(II)存在下的P(V)吸附动力学拟合参数
Table 4 The fitting parameter of P(V) adsorption kinetic when co-adsorbed with As(V) onto goethite
| 吸附质 Adsorbate | 准一级动力学 Pseudo-first-order | 准二级动力学 Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe1/ (μmol∙g-1) | k1/ min-1 | R2 | Qe2/ (μmol∙g-1) | k2/ (g∙μmol-1∙min-1) | R2 | ||
| P(V)1 | 216.14 | 0.5679 | 0.804 | 242.72 | 0.0012 | 0.999 | |
| P(V)2 | 130.69 | 0.3042 | 0.775 | 240.96 | 0.0010 | 0.999 | |
| P(V)3 | 402.49 | 0.5954 | 0.899 | 251.89 | 0.0041 | 0.999 | |
| P(V)4 | 174.82 | 0.6929 | 0.406 | 271.74 | 0.0003 | 0.999 | |
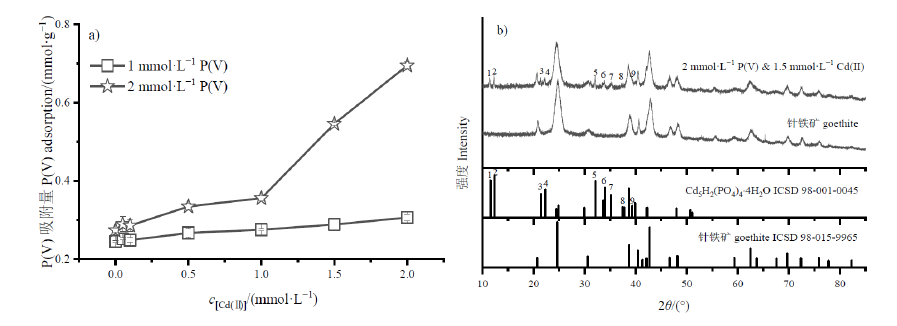
图5 不同浓度Cd(II)对P(V)吸附的影响(a);2 mmol∙L-1 P(V)与1.5 mmol∙L-1 Cd(II)在针铁矿上吸附的XRD图谱(b)
Figure 5 Effects of different Cd(II) concentrations on P(V) adsorption (a); XRD patterns (b) of 2 mmol∙L-1 P(V) and 1.5 mmol∙L-1 Cd(II) adsorbed onto goethite
| [1] |
ANTELO J, AVENA M, FIOL S, et al., 2005. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite-water interface[J]. Journal of Colloid and Interface Science, 285(2): 476-486.
DOI URL |
| [2] |
ATOUEI M T, RAHNEMAIE R, KALANPA E G, et al., 2016. Competitive adsorption of magnesium and calcium with phosphate at the goethite water interface: Kinetics, equilibrium and CD-MUSIC modeling[J]. Chemical Geology, 437(10): 19-29.
DOI URL |
| [3] | CORNELL R M, SCHWERTMANN U, 2003. The iron oxides: structure, properties, reactions, occurrences and uses[M]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA: 14-29, 531-535. |
| [4] | COSTA E T S, GUILHERME L R G, LOPES G, et al., 2012. Competitive sorption of arsenate and phosphate on aluminum mining by-product[J]. Water, Air, & Soil Pollution, 223(8): 5433-5444. |
| [5] |
ELZINGA E J, KRETZSCHMAR R, 2013 In situ ATR-FTIR spectroscopic analysis of the co-adsorption of orthophosphate and Cd(II) onto hematite[J]. Geochimica et Cosmochimica Acta, 117(9): 53-64.
DOI URL |
| [6] |
ESSINGTON M E, VERGEER K A, 2015. Adsorption of antimonate, phosphate, and sulfate by manganese dioxide: competitive effects and surface complexation modeling[J]. Soil Science Society of America Journal, 79(3): 803-814.
DOI URL |
| [7] | FLATEN D, SHARPLEY A, JARVIE H, et al., 2019. Reducing unintended consequences of agricultural phosphorus[J]. Better Crops Plant Food, 103(1): 33-35. |
| [8] |
HEALTHMAN G C, SHARPLEY A N, SMITH S J, et al., 1994. Land application of poultry litter application and water quality in Oklahoma, U.S.A.[J]. Fertilizer Research, 40(3): 165-173.
DOI URL |
| [9] |
JARVIE H P, SHARPLEY A N, FLATEN D, et al., 2019. Phosphorus mirabilis: Illuminating the past and future of phosphorus stewardship[J]. Journal of Environmental Quality, 48(5): 1127-1132.
DOI URL |
| [10] |
JARVIE H P, SHARPLEY A N, WITHERS P J A, et al., 2013. Phosphorus mitigation to control river eutrophication: Murky waters, inconvenient truths, and “postnormal” science[J]. Journal of Environmental Quality, 42(2): 295-304.
DOI URL |
| [11] |
LI L, STANFORTH R, 2000. Distinguishing adsorption and surface precipitation of phosphate on goethite (α-FeOOH)[J]. Journal of Colloid and Interface Science, 230(1): 12-21.
DOI URL |
| [12] |
LIU J, ZHU R L, XU T Y, et al., 2016 Co-adsorption of phosphate and zinc (II) on the surface of ferrihydrite[J]. Chemosphere, 144(2): 1148-1155.
DOI URL |
| [13] |
LIU Y T, HESTERBERG D, 2011. Phosphate bonding on non-crystalline Al/Fe-hydroxide coprecipitates[J]. Environmental Science & Technology, 45(15): 6283-6289.
DOI URL |
| [14] |
MANNING B A, GOLDBERG S, 1996. Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals[J]. Soil Science Society of America Journal, 60(1): 121-131.
DOI URL |
| [15] |
MEKONNEN M M, HOEKSTRA A Y, 2018. Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: A high-resolution global study[J]. Water Resources Research, 54(1): 345-358.
DOI URL |
| [16] |
PINTOR A M A, BRANDÃO C C, BOAVENTURA R A R, et al., 2021. Multicomponent adsorption of pentavalent As, Sb and P onto iron-coated cork granulates[J]. Journal of Hazardous Materials, DOI: 10.1016/j.jhazmat.2020.124339.
DOI |
| [17] |
QIU J, 2010. Phosphate fertilizer warning for China[J]. Nature, DOI: 10.1038/news.2010.498.
DOI |
| [18] |
RUSSELL J D, PARFITT R L, FRASER A R, et al., 1974. Surface structures of gibbsite goethite and phosphated goethite[J]. Nature, 248(5445): 220-221.
DOI URL |
| [19] |
SHARPLEY A, JARVIE H, FLATEN D, et al., 2018. Celebrating the 350th anniversary of phosphorus discovery: A conundrum of deficiency and excess[J]. Journal of Environmental Quality, 47(4): 774-777.
DOI URL |
| [20] |
SMITH D R, MACRAE M L, KLEINMAN P J A, et al., 2019. The latitudes, attitudes, and platitudes of watershed phosphorus management in North America[J]. Journal of Environmental Quality, 48(5): 1176-1190.
DOI URL |
| [21] | STOCKDALE E A, SHEPHERD M A, FORTUNE S, et al., 2002. Soil fertility in organic farming systems-fundamentally different?[J]. Soil Use and Management, 18(1): 301-308. |
| [22] |
TAO L, LI F B, WANG Y K, et al., 2010 Reductive activity of adsorbed Fe (II) on iron (oxyhydr) oxides for 2-nitrophenol transformation[J]. Clays and Clay Minerals, 58(5): 682-690.
DOI URL |
| [23] |
TAO L, WEN X C, LI H, et al., 2021. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment[J]. Applied Clay Science, 204: 106021.
DOI URL |
| [24] |
TEJEDOR-TEJEDOR M I, ANDERSON M A, 1990. The protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobility[J]. Langmuir, 6(3): 602-611.
DOI URL |
| [25] |
VIOLANTE A, PIGNA M, 2002. Competitive sorption of arsenate and phosphate on different clay minerals and soils[J]. Soil Science Society of America Journal, 66(6): 1788-1796.
DOI URL |
| [26] |
WEI S Y, TAN W F, LIU F, et al., 2014. Surface properties and phosphate adsorption of binary systems containing goethite and kaolinite[J]. Geoderma, 213(1): 478-484.
DOI URL |
| [27] |
YU N Y, WU K, TAO L, 2021. Synchronous reduction-fixation of reducible heavy metals from aqueous solutions: Application of novel mesoporous MFT/SBA-15 composite materials[J]. Chemosphere, 276: 130112.
DOI URL |
| [28] |
ZHANG W F, MA W Q, JI Y X, et al., 2008. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China[J]. Nutrient Cycling in Agroecosystems, 80(2): 131-144.
DOI URL |
| [29] |
ZHANG W F, TANG X M, FENG X H, et al., 2019. Management strategies to optimize soil phosphorus utilization and alleviate environmental risk in China[J]. Journal of Environmental Quality, 48(5): 1167-1175.
DOI URL |
| [30] |
ZHU M X, DING K Y, JIANG X, et al., 2007. Investigation on co-sorption and desorption of fluoride and phosphate in a red soil of China[J]. Water, Air, and Soil Pollution, 183(1): 455-465.
DOI URL |
| [31] | 黄国勤, 王兴祥, 钱海燕, 等, 2004. 施用化肥对农业生态环境的负面影响及对策[J]. 生态环境, 13(4): 656-660. |
| HUANG G Q, WANG X X, QIAN H Y, et al., 2004. Negative impact of inorganic fertilizes application on agricultural environment and its countermeasures[J]. Ecology and Environment, 13(4): 656-660. | |
| [32] |
黄敏雪, 管玉峰, 苏子贤, 等, 2022. 砷镉在不同矿物界面的相互作用过程[J/OL]. 土壤学报, DOI: 10.11766/trxb202101140027.
DOI |
|
HUANG M X, GUAN Y F, SU Z X, et al., 2022. Interfacial reactions between As(V) and Cd(II) co-adsorption onto various mineral surfaces[J/OL]. Acta Pedologica Sinica, DOI: 10.11766/trxb202101140027.
DOI |
|
| [33] | 吕贻忠, 李保国, 2006. 土壤学[M]. 北京: 中国农业出版社: 244-249. |
| LÜ Y Z, LI B G, 2006. Agrology[M]. Beijing: China Agriculture Press: 244-249. | |
| [34] | 全为民, 严力蛟, 2002. 农业面源污染对水体富营养化的影响及其防治措施[J]. 生态学报, 22(3): 291-299. |
| QUAN W M, YAN L J, 2002. Effects of agricultural non-point source pollution on eutrophication of water body and its control measure[J]. Acta Ecologica Sinica, 22(3): 291-299. | |
| [35] | 石华, 1989. 红壤研究四十春为庆祝建国40周年而作[J]. 土壤 (4): 174-179. |
| SHI H, 1989. Red soil research forty spring to celebrate the 40th anniversary of the founding of the people's Republic of China[J]. Soil (4): 174-179. | |
| [36] | 司友斌, 王慎强, 陈怀满, 2000. 农田氮、磷的流失与水体富营养化[J]. 土壤, 32(4): 188-193. |
| SI Y B, WANG S Q, CHEN H M, 2000. Loss of nitrogen and phosphorus in farmland and water eutrophication[J]. Soil, 32(4): 188-193. | |
| [37] | 王小玲, 马杰, 顾明华, 等, 2015. 砷和磷在不同污染类型土壤中的竞争吸附动力学[J]. 生态环境学报, 24(4): 694-699. |
| WANG X L, MA J, GU M H, et al., 2015. Competitive adsorption kinetics of arsenic and phosphorus in different kinds of contaminated soils[J]. Ecology and Environmental Sciences, 24(4): 694-699. | |
| [38] | 严玉鹏, 王小明, 熊娟, 等, 2020. 基于不同分析方法研究磷酸根在矿物表面吸附机制的进展[J]. 土壤学报, 57(1): 22-35. |
| YAN Y P, WANG X M, XIONG J, et al., 2020. Progresses in studies on sorption mechanisms of phosphate on minerals using multiple analytic approaches[J]. Acta Pedologica Sinica, 57(1): 22-35. |
| [1] | 王家一, 孙亭亭, 沙润钰, 谌婷红, 邢冉, 秦伯强, 施文卿. 富营养化湖泊蓝藻打捞减污降碳效果模拟研究[J]. 生态环境学报, 2023, 32(6): 1108-1114. |
| [2] | 杜丹丹, 高瑞忠, 房丽晶, 谢龙梅. 旱区盐湖盆地土壤重金属空间变异及对土壤理化因子的响应[J]. 生态环境学报, 2023, 32(6): 1123-1132. |
| [3] | 王超, 杨倩楠, 张池, 刘同旭, 张晓龙, 陈静, 刘科学. 丹霞山不同土地利用方式土壤磷组分特征及其有效性[J]. 生态环境学报, 2023, 32(5): 889-897. |
| [4] | 王铁铮, 瞿心悦, 刘春香, 李有志. 东江湖水质时空变化规律及其与流域土地利用的关系[J]. 生态环境学报, 2023, 32(4): 722-732. |
| [5] | 冯树娜, 吕家珑, 何海龙. KI淋洗对黄绵土汞污染的去除效果及土壤理化性状的影响[J]. 生态环境学报, 2023, 32(4): 776-783. |
| [6] | 陈敏毅, 朱航海, 佘伟铎, 尹光彩, 黄祖照, 杨巧玲. 珠三角某遗留造船厂场地土壤重金属人体健康风险评估及源解析[J]. 生态环境学报, 2023, 32(4): 794-804. |
| [7] | 张广毅, 张嘉涛, 王晓伟. 湖泊底泥微生物燃料电池中磷形态分布及释放研究[J]. 生态环境学报, 2023, 32(3): 590-598. |
| [8] | 樊慧琳, 张佳敏, 李欢, 王艳玲. 坡耕地稻田剖面磷的储存格局与流失风险研究[J]. 生态环境学报, 2023, 32(2): 283-291. |
| [9] | 杨瑞, 孙蔚旻, 李永斌, 郭丽芳, 焦念元. 尾矿先锋植物根际溶磷菌的分离鉴定与其促生研究[J]. 生态环境学报, 2023, 32(1): 166-174. |
| [10] | 肖洁芸, 周伟, 石佩琪. 土壤重金属含量高光谱反演[J]. 生态环境学报, 2023, 32(1): 175-182. |
| [11] | 黄伟佳, 刘春, 刘岳, 黄斌, 李定强, 袁再健. 南岭山地不同海拔土壤生态化学计量特征及影响因素[J]. 生态环境学报, 2023, 32(1): 80-89. |
| [12] | 黄宏, 郑欣芸, 李迎东, 赵旭, 俞锦辰, 汪振华. 大陈岛海域不同年龄褐菖鲉对重金属富集作用研究[J]. 生态环境学报, 2022, 31(9): 1885-1891. |
| [13] | 马闯, 王雨阳, 周通, 吴龙华. 污染土壤颗粒态有机质镉锌富集特征及其解吸行为研究[J]. 生态环境学报, 2022, 31(9): 1892-1900. |
| [14] | 李晓晖, 艾仙斌, 李亮, 王玺洋, 辛在军, 孙小艳. 新型改性稻壳生物炭材料对镉污染土壤钝化效果的研究[J]. 生态环境学报, 2022, 31(9): 1901-1908. |
| [15] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
