生态环境学报 ›› 2021, Vol. 30 ›› Issue (12): 2402-2410.DOI: 10.16258/j.cnki.1674-5906.2021.12.016
宋玥言1,2,3( ), 袁再健2,3, 黄斌2,3,*(
), 袁再健2,3, 黄斌2,3,*( ), 谢真越2,3, 刘永杰1
), 谢真越2,3, 刘永杰1
收稿日期:2021-08-10
出版日期:2021-12-18
发布日期:2022-01-04
通讯作者:
*黄斌(1986年生),男,助理研究员,博士,从事土壤污染物环境行为、水土保持与面源污染方面的研究工作。E-mail: bhuang@soil.gd.cn作者简介:宋玥言(1997年生),女,硕士研究生,主要从事土壤重金属污染与农业面源污染方面的研究。E-mail: 1071782063@qq.com
基金资助:
SONG Yueyan1,2,3( ), YUAN Zaijian2,3, HUANG Bin2,3,*(
), YUAN Zaijian2,3, HUANG Bin2,3,*( ), XIE Zhenyue2,3, LIU Yongjie1
), XIE Zhenyue2,3, LIU Yongjie1
Received:2021-08-10
Online:2021-12-18
Published:2022-01-04
摘要:
中国南方红壤区是Cd污染的集中地区,作为土壤修复材料的生物炭对Cd在土壤团聚体颗粒中的吸附行为具有重要的影响。为了研究生物炭对Cd在不同粒级团聚体中的吸附行为的影响规律,提高土壤修复过程中对不同粒径土壤团聚体改良的针对性,通过批量吸附实验,结合扫描电镜、FTIR红外等方法,探究了生物炭添加量(原土质量比例分别为0、1%、3%、5%、7%)、溶液初始pH值(2.0—6.5)等因素对不同粒径(>1 mm、1—0.25 mm、0.25—0.05 mm、<0.05 mm)红壤团聚体吸附Cd的影响。结果表明:生物炭添加能明显增加红壤中有机质、CEC以及游离氧化铁的含量;随生物炭添加比例的增加,Cd在土壤中的吸附量显著增加,生物炭添加量为原土比例的1%时,对Cd的单位吸附量增加最大,过高的生物炭投加量会降低生物炭的利用效率;Langmuir和Freundlich模型都能很好描述生物炭添加前后红壤团聚体中Cd的等温吸附特性(0.9730≤R2≤0.9961),生物炭添加对增加不同粒径团聚体吸附Cd能力的大小不同,总体上,土壤颗粒粒径越小对Cd的吸附能力越强,但是Cd在>1 mm团聚体中的吸附量相对增加量最大(42.9%);生物炭的添加能降低不同团聚体中Cd的解吸量,并随团聚体粒径的减小Cd的解吸量呈减小趋势,大粒径(>1 mm)团聚体中Cd的解吸量降低最为明显;同时,生物炭添加使团聚体Cd吸附量在较高pH情况下仍能有效增加,不同pH条件下,<0.05 mm的团聚体无论在生物炭添加前后均表现出最高的Cd吸附量,总体上,生物炭对于花岗岩发育的酸性红壤团聚体中的Cd具有较好的固定作用。
中图分类号:
宋玥言, 袁再健, 黄斌, 谢真越, 刘永杰. 生物炭对红壤团聚体吸附Cd的影响研究[J]. 生态环境学报, 2021, 30(12): 2402-2410.
SONG Yueyan, YUAN Zaijian, HUANG Bin, XIE Zhenyue, LIU Yongjie. Studies on the Influence of Biochar on the Adsorption of Cd onto Red Soil Aggregates[J]. Ecology and Environment, 2021, 30(12): 2402-2410.
| 处理 Treatment | 土壤粒径 Particle size/ mm | pH | 有机质质量分数 w(soil organic matter)/ (g∙kg-1) | 比表面积 Specific surface area/ (m2∙kg-1) | 阳离子交换量 Cation Exchange Capacity/ (cmol∙kg-1) | 游离氧化铁质量分数 w(free iron oxide)/ (g∙kg-1) |
|---|---|---|---|---|---|---|
| 0%原土质量比例生物炭 0% biochar addition | 原土 | 4.86 | 6.79 | 293.5 | 9.99 | 12.64 |
| >1 | 4.68 | 3.82 | 355.6 | 8.73 | 9.37 | |
| 1-0.25 | 4.87 | 8.94 | 339.6 | 7.99 | 8.12 | |
| 0.25-0.05 | 4.95 | 6.12 | 383.7 | 8.34 | 10.75 | |
| <0.05 | 5.08 | 9.50 | 450.8 | 14.17 | 16.86 | |
| 3%原土质量比例生物炭 3% biochar addition | 原土 | 5.09 | 14.52 | 339.9 | 12.40 | 14.27 |
| >1 | 5.25 | 11.17 | 376.9 | 9.86 | 10.71 | |
| 1-0.25 | 5.31 | 14.57 | 344.7 | 8.49 | 10.52 | |
| 0.25-0.05 | 5.20 | 16.75 | 439.1 | 9.03 | 14.35 | |
| <0.05 | 5.19 | 18.98 | 473.5 | 14.36 | 17.11 |
表1 原土和不同粒径团聚体的基本性质
Table 1 Basic properties of bulk soil and aggregates of different particle-size fractions
| 处理 Treatment | 土壤粒径 Particle size/ mm | pH | 有机质质量分数 w(soil organic matter)/ (g∙kg-1) | 比表面积 Specific surface area/ (m2∙kg-1) | 阳离子交换量 Cation Exchange Capacity/ (cmol∙kg-1) | 游离氧化铁质量分数 w(free iron oxide)/ (g∙kg-1) |
|---|---|---|---|---|---|---|
| 0%原土质量比例生物炭 0% biochar addition | 原土 | 4.86 | 6.79 | 293.5 | 9.99 | 12.64 |
| >1 | 4.68 | 3.82 | 355.6 | 8.73 | 9.37 | |
| 1-0.25 | 4.87 | 8.94 | 339.6 | 7.99 | 8.12 | |
| 0.25-0.05 | 4.95 | 6.12 | 383.7 | 8.34 | 10.75 | |
| <0.05 | 5.08 | 9.50 | 450.8 | 14.17 | 16.86 | |
| 3%原土质量比例生物炭 3% biochar addition | 原土 | 5.09 | 14.52 | 339.9 | 12.40 | 14.27 |
| >1 | 5.25 | 11.17 | 376.9 | 9.86 | 10.71 | |
| 1-0.25 | 5.31 | 14.57 | 344.7 | 8.49 | 10.52 | |
| 0.25-0.05 | 5.20 | 16.75 | 439.1 | 9.03 | 14.35 | |
| <0.05 | 5.19 | 18.98 | 473.5 | 14.36 | 17.11 |
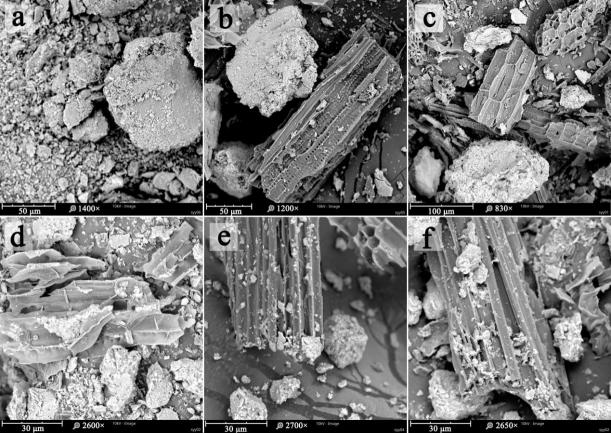
图1 原土(a)与3%原土质量比例生物炭添加下原土(b)和不同粒径土壤团聚体>1 mm(c)、1—0.25 mm(d)、0.25—0.05 mm(e)、<0.05 mm(f)扫描电子显微镜图
Fig.1 SEM images of bulk soil (a) and bulk soil, >1 mm (c), 1-0.25 mm (d), 0.25-0.05 mm (e)and<0.05 mm (f) aggregates amended with 3% biochar of original soil

图3 生物炭添加量对土壤吸附Cd的影响 T-0、T-1、T-3、T-5和T-7分别是向原土中添加原土比例0、1%、3%、5%和7%的生物炭
Fig. 3 Influence of biochar addition amount on Cd adsorption in soil T-0, T-1, T-3, T-5 and T-7 were biochar with 0, 1%, 3%, 5% and 7% of the original soil, respectively
| 生物炭比例 Biochar addition/ % | Langmuir方程 Langmuir equation | Freundlich方程 Freundlichr equation | |||||
|---|---|---|---|---|---|---|---|
| KL/ (L∙mg-1) | qm/ (mg∙kg-1) | R2 | KF/ (L∙kg-1) | n-1 | R2 | ||
| 0 | 0.003 | 1956.417 | 0.964 | 448.217 | 0.457 | 0.981 | |
| 1 | 0.013 | 3065.906 | 0.964 | 537.654 | 0.323 | 0.948 | |
| 3 | 0.019 | 3454.713 | 0.958 | 636.806 | 0.304 | 0.930 | |
| 5 | 0.018 | 3495.683 | 0.960 | 921.814 | 0.278 | 0.923 | |
| 7 | 0.018 | 3644.487 | 0.974 | 846.603 | 0.211 | 0.949 | |
表2 Langmuir和Freunlich方程拟合不同比例生物炭添加后土壤中Cd吸附结果
Table 2 Langmuir and Freundlich model fitting results for the adsorption results of Cd in red soil amended with biochar of different proportions
| 生物炭比例 Biochar addition/ % | Langmuir方程 Langmuir equation | Freundlich方程 Freundlichr equation | |||||
|---|---|---|---|---|---|---|---|
| KL/ (L∙mg-1) | qm/ (mg∙kg-1) | R2 | KF/ (L∙kg-1) | n-1 | R2 | ||
| 0 | 0.003 | 1956.417 | 0.964 | 448.217 | 0.457 | 0.981 | |
| 1 | 0.013 | 3065.906 | 0.964 | 537.654 | 0.323 | 0.948 | |
| 3 | 0.019 | 3454.713 | 0.958 | 636.806 | 0.304 | 0.930 | |
| 5 | 0.018 | 3495.683 | 0.960 | 921.814 | 0.278 | 0.923 | |
| 7 | 0.018 | 3644.487 | 0.974 | 846.603 | 0.211 | 0.949 | |
| 粒径 Particle size/mm | Langmuir方程 Langmuir equation | Freundlich方程 Freundlich equation | ||||||
|---|---|---|---|---|---|---|---|---|
| KL/(L∙mg-1) | qm/(mg∙kg-1) | R2 | KF/(L∙kg-1) | n-1 | R2 | |||
| 0%原土质量比例生物炭 0% biochar addition | >1 | 0.153 | 1956.895 | 0.995 | 202.038 | 0.059 | 0.986 | |
| 1-0.25 | 0.118 | 2058.161 | 0.996 | 184.337 | 0.085 | 0.989 | ||
| 0.25-0.05 | 0.108 | 2212.626 | 0.996 | 193.101 | 0.085 | 0.991 | ||
| <0.05 | 0.127 | 2288.936 | 0.995 | 187.057 | 0.089 | 0.990 | ||
| 3%原土质量比例生物炭 3% biochar addition | >1 | 0.134 | 2797.148 | 0.994 | 242.589 | 0.086 | 0.993 | |
| 1-0.25 | 0.121 | 2799.889 | 0.995 | 245.523 | 0.101 | 0.989 | ||
| 0.25-0.05 | 0.112 | 2839.222 | 0.996 | 207.824 | 0.083 | 0.992 | ||
| <0.05 | 0.201 | 3175.737 | 0.980 | 281.723 | 0.106 | 0.983 | ||
表3 Langmuir和Freundlich方程拟合未添加(0%)和3%生物炭添加条件下不同粒径团聚体中Cd吸附结果
Table 3 Langmuir and Freundlich model fitting results for the adsorption results of Cd in different soil particles amended with 0% and 3% biochar
| 粒径 Particle size/mm | Langmuir方程 Langmuir equation | Freundlich方程 Freundlich equation | ||||||
|---|---|---|---|---|---|---|---|---|
| KL/(L∙mg-1) | qm/(mg∙kg-1) | R2 | KF/(L∙kg-1) | n-1 | R2 | |||
| 0%原土质量比例生物炭 0% biochar addition | >1 | 0.153 | 1956.895 | 0.995 | 202.038 | 0.059 | 0.986 | |
| 1-0.25 | 0.118 | 2058.161 | 0.996 | 184.337 | 0.085 | 0.989 | ||
| 0.25-0.05 | 0.108 | 2212.626 | 0.996 | 193.101 | 0.085 | 0.991 | ||
| <0.05 | 0.127 | 2288.936 | 0.995 | 187.057 | 0.089 | 0.990 | ||
| 3%原土质量比例生物炭 3% biochar addition | >1 | 0.134 | 2797.148 | 0.994 | 242.589 | 0.086 | 0.993 | |
| 1-0.25 | 0.121 | 2799.889 | 0.995 | 245.523 | 0.101 | 0.989 | ||
| 0.25-0.05 | 0.112 | 2839.222 | 0.996 | 207.824 | 0.083 | 0.992 | ||
| <0.05 | 0.201 | 3175.737 | 0.980 | 281.723 | 0.106 | 0.983 | ||
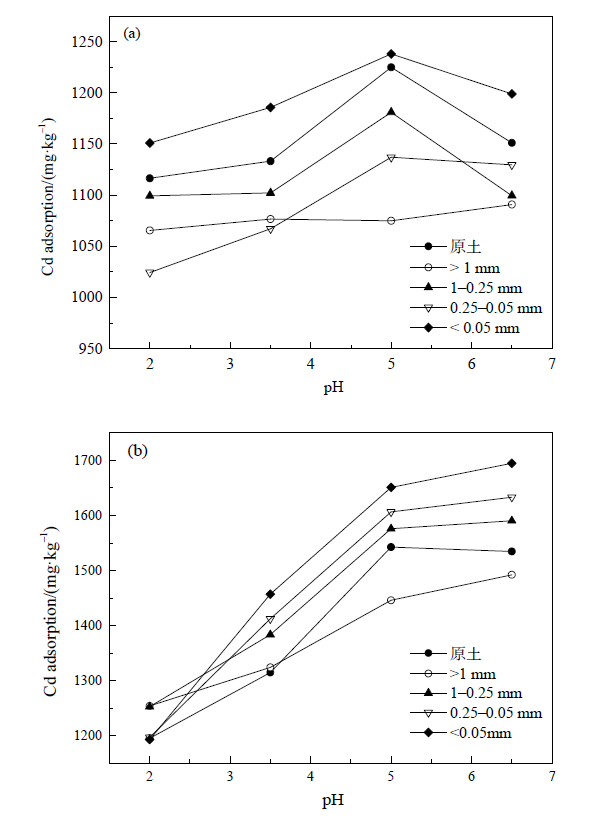
图6 不同初始 pH 条件下原土(a)和3%生物炭添加(b)土壤中Cd吸附量
Fig. 6 Adsorption amounts Cd in bulk soil (a) and soil amended with 3% biochar (b) under different initial pH value conditions
| [1] | ABDU N, MOHAMMED I, 2016. Adsorption-solubility equilibria and speciation of Pb, Cd, and Zn in a savanna soil[J]. Spanish Journal of Soilence, 6: 244-260. |
| [2] |
AJMONE-MARSAN F, BIASIOLI M, KRALJ T, et al., 2008. Metals in particle-size fractions of the soils of five European cities[J]. Environmental Pollution, 152(1): 73-81.
DOI URL |
| [3] |
BEGUERÍA S, ANGULO-MARTÍNEZ M, GASPAR L, 2015. Detachment of soil organic carbon by rainfall splash: Experimental assessment on three agricultural soils of Spain[J]. Geoderma, 245-246: 21-30.
DOI URL |
| [4] |
BRONICK C J, LAL R, 2005. Soil structure and management: a review[J]. Geoderma, 124(1-2): 3-22.
DOI URL |
| [5] |
CHEN X, CHEN G, CHEN L, et al., 2011. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution[J]. Bioresource Technology, 102(19): 8877-8884.
DOI URL |
| [6] |
EMMA M, 2006. Putting the carbon back: black is the new green[J]. Nature, 442(7103): 624-626.
DOI URL |
| [7] |
GILES CH, SMITH D, HUITSON A, et al., 1974. A general treatment and classification of the solute adsorption isotherm. I. Theoretical[J]. Journal of Colloid and Interface Science, 47(3): 755-765.
DOI URL |
| [8] |
GONG C, MA L, CHENG H, et al., 2014. Characterization of the particle size fraction associated heavy metals in tropical arable soils from Hainan Island, China[J]. Journal of Geochemical Exploration, 139: 109-114.
DOI URL |
| [9] |
GUPTA A, MUMTAZ S, LI C H, et al., 2019. Combatting antibiotic- resistant bacteria using nanomaterials[J]. Chemical Society Reviews, 48(2): 415-427.
DOI URL |
| [10] |
HAYNES R J, NAIDU R, 1998. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review[J]. Nutrient Cycling in Agroecosystems, 51(2): 123-137.
DOI URL |
| [11] |
HUANG B, YUAN Z J, LI D Q, et al., 2019. Loss characteristics of Cd in soil aggregates under simulated rainfall conditions[J]. Science of the Total Environment, 650(Pt 1): 313-320.
DOI URL |
| [12] | HUANG B, LI Z W, 2015. Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil[J]. Environmental Science & Pollution Research, 22(15): 11467-11477. |
| [13] |
LI J R, XU Y M, 2017. Immobilization remediation of Cd-polluted soil with different water condition[J]. Journal of Environmental Management, 193(15): 607-612.
DOI URL |
| [14] | LIANG A Z, YANG X M, ZHANG X P, et al., 2009. Soil organic carbon changes in particle-size fractions following cultivation of Black soils in China[J]. Soil & Tillage Research, 105(1): 21-26. |
| [15] | NELSON D W, 1996. Total carbon, organic carbon, and organic matter[J]. Methods of Soil Analysis, 9: 961-1010. |
| [16] |
RAO M M, RAO G, SESHAIAH K. et al., 2008. Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions[J]. Waste Management, 28(5): 849-858.
DOI URL |
| [17] |
SARKAR D, DE D K, DAS R, et al., 2014. Removal of organic matter and oxides of iron and manganese from soil influences boron adsorption in soil[J]. Geoderma, 214-215: 213-216.
DOI URL |
| [18] | TRAKAL L, M KOMÁREK, J SZÁKOVÁ, et al., 2011. Biochar application to metal-contaminated soil: Evaluating of Cd, Cu, Pb and Zn sorption behavior using single- and multi-element sorption experiment[J]. Plant Soil & Environment, 57(8): 372-380. |
| [19] |
WANG Y, TANG X W, CHEN Y M, et al., 2009. Adsorption behavior and mechanism of Cd(II) on loess soil from China [J]. Journal of Hazardous Materials, 172(1): 30-37.
DOI URL |
| [20] | 白庆中, 宋燕光, 王晖, 等, 2000. 有机物对重金属在粘土中吸附行为的影响[J]. 环境科学, 21(5): 64-67. |
|
BAI Q Z, SONG Y G, WANG H, et al., 2000. Effect of organic acids on heavy metal migration in clay[J]. Environmental Science, 21(5): 64-67.
DOI URL |
|
| [21] | 鲍士旦, 2000. 土壤农化分析[M]. 北京: 中国农业出版社. |
| BAO S D, 2000. Soil and agricultural chemistry analysis[M]. Beijing: China Agriculture Press. | |
| [22] | 陈晶中, 陈杰, 谢学俭, 等, 2003. 土壤污染及其环境效应[J]. 土壤, 35(4): 298-303. |
| CHEN J Z, CHEN J, XIE X J, et al., 2003. Soil pollution and its environmental Impact[J]. Soils, 35(4): 298-303. | |
| [23] | 陈再明, 陈宝梁, 周丹丹, 等, 2013. 水稻秸秆生物碳的结构特征及其对有机污染物的吸附性能[J]. 环境科学学报, 33(1): 9-19. |
| CHEN Z M, CHEN B L, ZHOU D D, et al., 2013. Composition and sorption properties of rice-straw derived biochars[J]. Acta Scientiae Circumstantiae, 33(01): 9-19. | |
| [24] | 陈能场, 郑煜基, 何晓峰, 等, 2017. 《全国土壤污染状况调查公报》探析[J]. 农业环境科学学报, 36(9): 1689-1692. |
| CHEN N C, ZHENG Y J, HE X F, et al., 2017. Analysis of the Report on the national general survey of soil contamination[J]. Journal of Agro-Environment Science, 36(9): 1689-1692. | |
| [25] | 洪舒蔓, 夏建国, 张世熔, 等, 2010. 名山河流域水稻土组分对微团聚体吸附-解吸铜的影响[J]. 环境科学学报, 30(3): 578-586. |
| HONG S M, XIA J G, ZHANG S R, et al., 2010. Effect of paddy soil components on adsorption and desorption of copper bymicroaggregates in paddy soil from the Mingshan watershed[J]. Acta Scientiae Circumstantiae, 30(3): 578-586. | |
| [26] | 李江舟, 代快, 张立猛, 等, 2016. 施用生物炭对云南烟区红壤团聚体组成及有机碳分布的影响[J]. 环境科学学报, 36(6): 2114-2120. |
| LI J Z, DAI K, ZHANG L M, et al., 2016. Effects of biochar application on soil organic carbon distribution and soilaggregate composition of red soils in Yunnan tobacco planting area[J]. Acta Scientiae Circumstantiae, 36(6): 2114-2120. | |
| [27] | 李力, 陆宇超, 刘娅, 等, 2012. 玉米秸秆生物炭对Cd(Ⅱ)的吸附机理研究[J]. 农业环境科学学报, 31(11): 2277-2283. |
| LI L, LU Y C, LIU Y, et al., 2012. Adsorption Mechanisms of Cadmium(Ⅱ) on Biochars Derived from Corn Straw[J]. Journal of Agro-Environment Science, 31(11): 2277-2283. | |
| [28] | 刘莹莹, 秦海芝, 李恋卿, 等, 2012. 不同作物原料热裂解生物质炭对溶液中Cd2+和Pb2+的吸附特性[J]. 生态环境学报, 21(1): 146-152. |
| LIU Y Y, QIN H Z, LI L Q, et al., 2012. Adsorption of Cd2+ and Pb2+ in aqueous solution by biochars produced from the pyrolysis of different crop feedstock[J]. Ecology and Environmental Sciences, 21(1): 146-152. | |
| [29] | 孟祥天, 蒋瑀霁, 王晓玥, 等, 2018. 生物质炭和秸秆长期还田对红壤团聚体和有机碳的影响[J]. 土壤, 50(2): 326-332. |
| MENG X T, JIANG Y J, WANG X Y, et al., 2018. Effects of long-term application of biochar and straws on red soil aggregate compostion and organic carbon distribution[J]. Soils, 50(2): 326-332. | |
| [30] | 尚杰, 耿增超, 赵军, 等, 2015. 生物炭对塿土水热特性及团聚体稳定性的影响[J]. 应用生态学报, 26(7): 1969-1976. |
| SHANG J, GENG Z C, ZHAO J, et al., 2015. Effects of biochar on water thermal properties and aggregate stability of Lou soil[J]. Chinese Journal of Applied Ecology, 26(7): 1969-1976. | |
| [31] | 王冰, 赵闪闪, 秦治家, 等, 2016. 生物质炭对黑土吸附-解吸硝态氮性能的影响[J]. 农业环境科学学报, 35(1): 115-121. |
| WANG B, ZHAO S S, QIN Z J, et al., 2016. Effect of biochar on adsorption-desorption characteristics of nitrate nitrogen in black soil[J]. Journal of Agro-Environment Science, 35(1): 115-121. | |
| [32] | 王芳, 2008. 水稻土团聚体颗粒组对外源污染物(镉、铜和菲)的吸附-解吸特性研究[D]. 南京: 南京农业大学. |
| WANG F, 2008. Sorption-desorption of cadmium,copper and phenanthrene by size fractions of microaggregates from paddy soils[D]. Nanjing: Nanjing Agricultural University. | |
| [33] | 王亚琼, 2019. 生物炭对土壤团聚体和钾素的影响[D]. 杨凌: 中国科学院大学 (中国科学院教育部水土保持与生态环境研究中心). |
| WANG Y Q, 2019. Effect of biochar on soil aggregate and potssium[D]. Yangling: Research Center of Soil and Water Conservation and Ecological Environment, Chinese Academy of Sciences and Ministry of Education. | |
| [34] | 武玉, 徐刚, 吕迎春, 等, 2014. 生物炭对土壤理化性质影响的研究进展[J]. 地球科学进展, 29(1): 68-79. |
| WU Y, XU G, LV Y C, et al., 2014. Effects of Biochar Amendment on Soil Physical and Chemical Properties: Current Status and Knowledge Gaps[J]. Advances in Earth Science, 29(1): 68-79. | |
| [35] | 许海波, 赵道远, 刘培亚, 等, 2013. 磷酸盐对水稻土团聚体不同类型重金属镉、铬(Ⅵ)吸附的影响[J]. 生态环境学报, 22(5): 857-862. |
| XU H B, ZHAO D Y, LIU P Y, et al., 2013. Effect of phosphate on the kinetic of the adsorption of different types of heavy metal: Cadmium and chromium by aggregates in paddy soil[J]. Ecology and Environmental Sciences, 22(05): 857-862. | |
| [36] | 周涵君, 韩秋静, 马静, 等, 2019. 生物炭对红壤和褐土中镉形态的影响[J]. 植物营养与肥料学报, 25(3): 433-442. |
| ZHOU H J, HAN Q J, MA J, et al., 2019. Effects of biochar on Cd forms in red soil and cinnamon soil[J]. Journal of Plant Nutrition and Fertilizers, 25(3): 433-442. |
| [1] | 李传福, 朱桃川, 明玉飞, 杨宇轩, 高舒, 董智, 李永强, 焦树英. 有机肥与脱硫石膏对黄河三角洲盐碱地土壤团聚体及其有机碳组分的影响[J]. 生态环境学报, 2023, 32(5): 878-888. |
| [2] | 周沁苑, 董全民, 王芳草, 刘玉祯, 冯斌, 杨晓霞, 俞旸, 张春平, 曹铨, 刘文亭. 放牧方式对高寒草地瑞香狼毒根际土壤团聚体及有机碳特征的影响[J]. 生态环境学报, 2023, 32(4): 660-667. |
| [3] | 赵维彬, 唐丽, 王松, 刘玲玲, 王树凤, 肖江, 陈光才. 两种生物炭对滨海盐碱土的改良效果[J]. 生态环境学报, 2023, 32(4): 678-686. |
| [4] | 赵良侠, 高坤, 黄婷婷, 高也, 琚唐丹, 蒋秋阳, 金珩, 熊蕾, 汤在琳, 高灿红. 玉米籽粒高/低镉积累自交系不同生育期的镉累积特性研究[J]. 生态环境学报, 2023, 32(4): 766-775. |
| [5] | 杨耀东, 陈玉梅, 涂鹏飞, 曾清如. 经济作物轮作模式下镉污染农田修复潜力[J]. 生态环境学报, 2023, 32(3): 627-634. |
| [6] | 樊慧琳, 张佳敏, 李欢, 王艳玲. 坡耕地稻田剖面磷的储存格局与流失风险研究[J]. 生态环境学报, 2023, 32(2): 283-291. |
| [7] | 徐敏, 许超, 余光辉, 尹力初, 张泉, 朱捍华, 朱奇宏, 张杨珠, 黄道友. 地下水位和长期秸秆还田对土壤镉有效性及稻米镉含量的影响[J]. 生态环境学报, 2023, 32(1): 150-157. |
| [8] | 崔远远, 张征云, 刘鹏, 张运春, 张桥英. 镉与聚乙烯微塑料胁迫对小白菜根系的形态特征和分形维数的影响[J]. 生态环境学报, 2023, 32(1): 158-165. |
| [9] | 向兴, 满百膺, 张俊忠, 罗洋, 毛小涛, 张超, 孙丙华, 王希. 黄山土壤细菌群落及氮循环功能群的垂向分布格局[J]. 生态环境学报, 2023, 32(1): 56-69. |
| [10] | 游宏建, 张文文, 兰正芳, 马兰, 张宝娣, 穆晓坤, 李文慧, 曹云娥. 蚯蚓原位堆肥与生物炭对黄瓜根结线虫及根际微生物的影响[J]. 生态环境学报, 2023, 32(1): 99-109. |
| [11] | 李晓晖, 艾仙斌, 李亮, 王玺洋, 辛在军, 孙小艳. 新型改性稻壳生物炭材料对镉污染土壤钝化效果的研究[J]. 生态环境学报, 2022, 31(9): 1901-1908. |
| [12] | 李秀华, 赵玲, 滕应, 骆永明, 黄标, 刘冲, 刘本乐, 赵其国. 贵州汞矿区周边农田土壤汞镉复合污染特征空间分布及风险评估[J]. 生态环境学报, 2022, 31(8): 1629-1636. |
| [13] | 陶玲, 黄磊, 周怡蕾, 李中兴, 任珺. 污泥-凹凸棒石共热解生物炭对矿区土壤重金属生物有效性和环境风险的影响[J]. 生态环境学报, 2022, 31(8): 1637-1646. |
| [14] | 房献宝, 张智钧, 赖阳晴, 叶脉, 刁增辉. 新型污泥生物炭对土壤重金属Cr和Cd的修复研究[J]. 生态环境学报, 2022, 31(8): 1647-1656. |
| [15] | 钱莲文, 余甜甜, 梁旭军, 王义祥, 陈永山. 茶园土壤酸化改良中生物炭应用5 a后的稳定性研究[J]. 生态环境学报, 2022, 31(7): 1442-1447. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
