生态环境学报 ›› 2021, Vol. 30 ›› Issue (11): 2257-2266.DOI: 10.16258/j.cnki.1674-5906.2021.11.017
收稿日期:2021-01-10
出版日期:2021-11-18
发布日期:2021-12-29
通讯作者:
* 蒋海明(1977年生),男,教授,主要研究方向为应用环境微生物、生物能源、环境污染物的生物治理及监测。E-mail: jhmhn@163.com作者简介:李侠(1979年生),女(满族),副教授,研究方向为环境微生物与微生物冶金。E-mail: lixia2002hn@163.com
基金资助:
LI Xia1( ), LAN Jianying2,3, JIANG Haiming2,3,*(
), LAN Jianying2,3, JIANG Haiming2,3,*( )
)
Received:2021-01-10
Online:2021-11-18
Published:2021-12-29
摘要:
甲烷(CH4)是一种温室气体,其温室效应是二氧化碳的26倍。减少甲烷排放,可以缓解温室效应,有助于保护大气环境。微生物介导的甲烷厌氧氧化是调节甲烷向大气排放的关键过程。金属氧化物或金属离子可以作为甲烷厌氧氧化的电子受体,且金属依赖的甲烷厌氧氧化是减小全球甲烷排放的一个重要途径。虽然研究表明金属依赖型厌氧甲烷营养古菌可以促进这一过程,但其菌种、代谢途径及胞外电子传递途径尚缺乏深入研究。文章从金属依赖型厌氧甲烷氧化菌、金属依赖型厌氧甲烷氧化机制及金属依赖型厌氧甲烷氧化菌胞外电子传递机制等方面对金属依赖型厌氧甲烷氧化研究现状进行了概述,分析了金属依赖型厌氧甲烷氧化研究存在的问题,并讨论了其今后的研究方向,为金属依赖型厌氧甲烷氧化研究提供参考。
中图分类号:
李侠, 兰建英, 蒋海明. 金属依赖型厌氧甲烷氧化研究进展[J]. 生态环境学报, 2021, 30(11): 2257-2266.
LI Xia, LAN Jianying, JIANG Haiming. Advance in Metal Iron-dependent Anaerobic Oxidation of Methane[J]. Ecology and Environment, 2021, 30(11): 2257-2266.
| 金属 | 主要功能微生物 | 文献 |
|---|---|---|
| 铁(III) | ANME-1, ANME-2及ANME-3 | Chang et al., |
| ANME-2a和ANME-2c | Scheller et al., | |
| AAA | Ettwig et al., | |
| M. acetivorans C2A | Soo et al., Yan et al., | |
| Ca. M. ferrireducens | Cai et al., | |
| 锰(Ⅳ) | Ca. M. manganicus, Ca. M. manganireducens | Leu et al., |
| 硒 (VI) | Ca. Methanoperedens, Ca. Methylomirabilis | Luo et al., |
| Methylomonas | Lai et al., | |
| 钒(Ⅴ) | Methylomonas, Methanobacterium, Stenotrophomonas | Zhang et al., |
| 锑(V) | Methanosarcina和Sb(V)-reducing microbe (unknown) | Lai et al., |
| 铬(VI) | ANME-2d和/或Cr(VI)-reducing microbe (unknown) | Lu et al., |
| Methanobacterium, Methanosarcina, Meiothermus, ANME-2d | Dong et al., | |
| Ca. Methanoperedens和/或Cr(VI)-reducing microbe (unknown) | Luo et al., | |
| Methylococcus capsulatus (Bath) | Al-Hasin et al., | |
| 砷(V) | ANME-1和ANME-2a-c | Shi et al., |
表1 文献报道的金属依赖型厌氧甲烷氧化微生物
Table 1 Microbes reported as M-DAOM
| 金属 | 主要功能微生物 | 文献 |
|---|---|---|
| 铁(III) | ANME-1, ANME-2及ANME-3 | Chang et al., |
| ANME-2a和ANME-2c | Scheller et al., | |
| AAA | Ettwig et al., | |
| M. acetivorans C2A | Soo et al., Yan et al., | |
| Ca. M. ferrireducens | Cai et al., | |
| 锰(Ⅳ) | Ca. M. manganicus, Ca. M. manganireducens | Leu et al., |
| 硒 (VI) | Ca. Methanoperedens, Ca. Methylomirabilis | Luo et al., |
| Methylomonas | Lai et al., | |
| 钒(Ⅴ) | Methylomonas, Methanobacterium, Stenotrophomonas | Zhang et al., |
| 锑(V) | Methanosarcina和Sb(V)-reducing microbe (unknown) | Lai et al., |
| 铬(VI) | ANME-2d和/或Cr(VI)-reducing microbe (unknown) | Lu et al., |
| Methanobacterium, Methanosarcina, Meiothermus, ANME-2d | Dong et al., | |
| Ca. Methanoperedens和/或Cr(VI)-reducing microbe (unknown) | Luo et al., | |
| Methylococcus capsulatus (Bath) | Al-Hasin et al., | |
| 砷(V) | ANME-1和ANME-2a-c | Shi et al., |
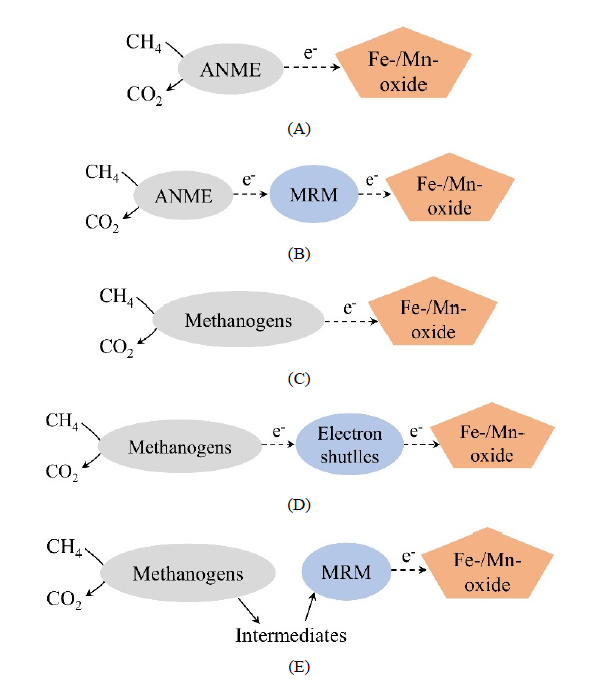
图2 微生物介导的M-DAOM不同机制(He et al.,2018;何丹等,2020) (A)ANME单独完成M-DAOM M-DAOM by ANME alone;(B)ANME与合作伙伴MRM共同完成M-DAOM M-DAOM by cooperation between ANME and partner MRM;(C)产甲烷古菌单独完成M-DAOM M-DAOM by archaea methanogens alone;(D)产甲烷古菌与电子穿梭体完成M-DAOM M-DAOM by archaea methanogens and electron shuttle;(E)产甲烷古菌和MRM通过甲烷代谢中间产物完成M-DAOM M-DAOM by archaea methanogens and MRM through intermediate products of methane metabolism。ANME:厌氧甲烷营养古菌 anaerobic methanotrophic archaea;MRM:金属还原微生物 metal-reducing microorganisms
Fig. 2 Different mechanisms of microbe-mediated M-DAOM (He et al., 2018; He et al., 2020)
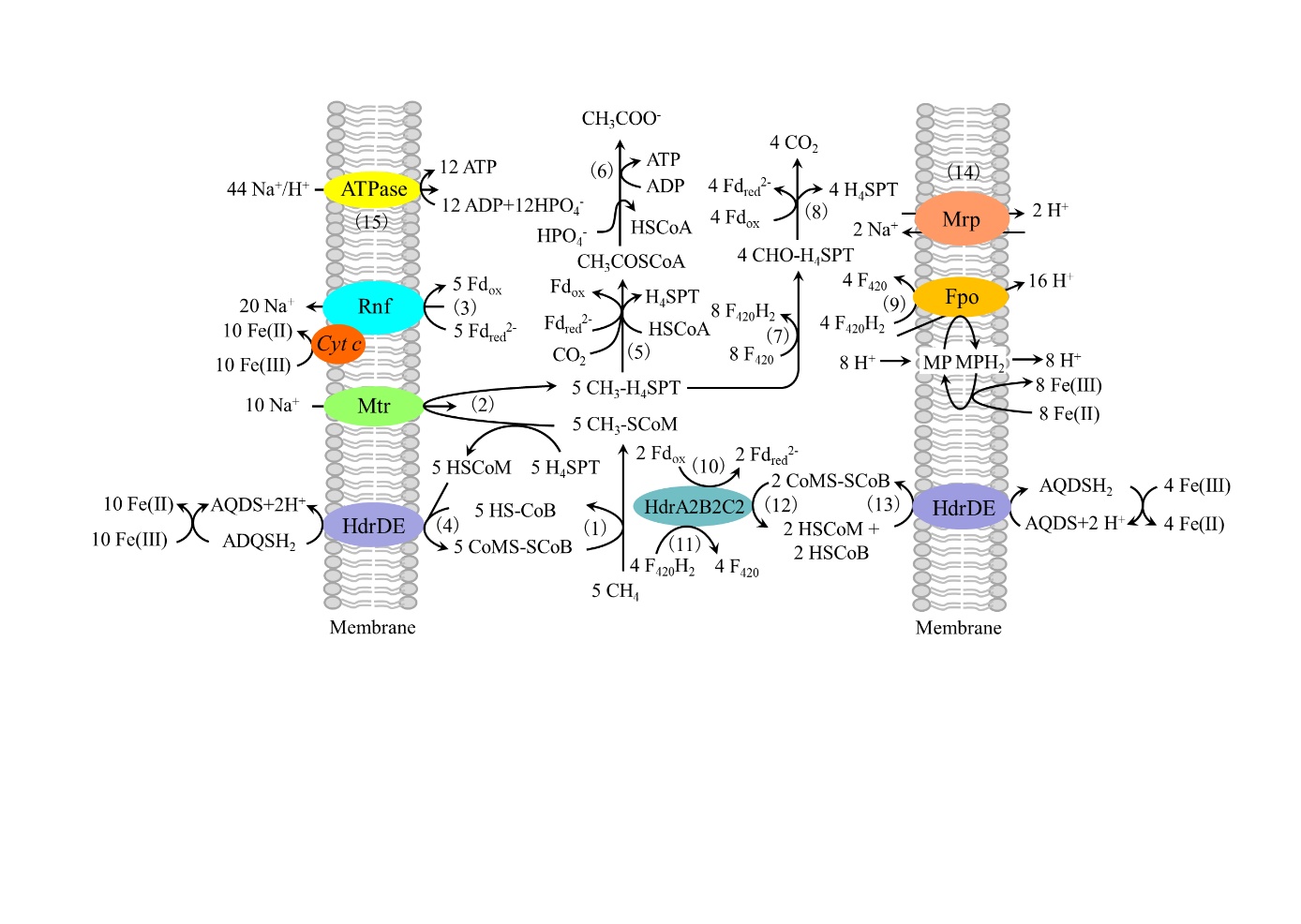
图3 M. acetivorans C2A Fe(Ⅲ)依赖的甲烷氧化及胞外电子传递假设途径(Yan et al.,2018) CO脱氢酶/乙酰辅酶A合成酶 CO dehydrogenase/acetyl-CoA synthase(反应5);乙酸激酶和磷酸转乙酰酶 acetate kinase and phosphotransacetylase(反应6);辅酶F420依赖的亚甲基四氢八叠蝶呤还原酶 coenzyme F420 (F420)-dependent methylenetetrahydrosarcinapterin (H4SPT) reductase、辅酶F420依赖的亚甲基四氢八叠蝶呤脱氢酶 F420-dependent methylene-H4SPT dehydrogenase、甲基四氢八叠蝶呤环化水解酶 methenyl-H4SPT cyclohydrolase、甲酰甲烷呋喃:四氢八叠蝶呤甲酰转移酶 formylmethanofuran H4SPT formyltransferase(反应7);甲酰甲烷呋喃脱氢酶 formylmethanofuran dehydrogenase(反应8)。Fwd:甲酰甲烷呋喃脱氢酶 formyl-methanofuran dehydrogenase;Ftr:甲酰甲烷呋喃/四氢八叠蝶呤甲酰转移酶 formylmethanofuran/H4MPT formyltransferase;Mch:甲基四氢八叠蝶呤环化水解酶 methenyl-H4MPT cyclohydrolase;Mtd:辅酶F420依赖的亚甲基四氢八叠蝶呤脱氢酶 F420-dependent methylene-H4MPT dehydrogenase;Mer:F420依赖的亚甲基四氢八叠蝶呤还原酶 F420-dependent methylene-H4MPT reductase;Mtr:转运Na+的甲基四氢甲烷蝶呤:辅酶M甲基转移酶 Na+-translocating methyl-H4MPT:coenzyme M methyltransferase;Mcr:甲基辅酶M甲基还原酶 methyl-coenzyme M reductase;Fpo:还原态辅酶F420脱氢酶 F420H2 dehydrogenase;ATPase:腺苷三磷酸合成酶 ATP synthetase;HdrDE:异二硫化物还原酶亚单位D和E heterodisulfide reductase subunits D and E;HdrA2B2C2:异二硫化物还原酶亚单位A2、B2和C2 heterodisulfide reductase subunits A2, B2, and C2;Rnf:Rnf 复合体 Rnf complex;Mrp:Na+/H+逆向转运体 Na+/H+ antiportor;MP:氧化态甲烷吩嗪 oxidized methanophenazine;MPH2:还原态甲烷吩嗪 reduced methanophenazine;H4MPT:四氢甲烷蝶呤 tetrahydromethanopterin;MFR:甲烷呋喃 methanofuran;cyt c:c型细胞色素 c-type cytochrome;F420H2:还原态辅酶F420 reduced coenzyme F420;F420:氧化态辅酶F420 oxidized coenzyme F420;CoB-SH:辅酶B coenzyme B;CoM-SH:辅酶M coenzyme M;CoM-S-S-CoB:辅酶M和辅酶B的异二硫化物 Heterodisulfide of coenzyme M and coenzyme B;Fdox:氧化态铁氧化还原蛋白 oxidized ferredoxin;Fdred-:一个电子还原的铁氧化还原蛋白 one-electron reduced ferredoxin;Fdred2-:完全还原的铁氧化还原蛋白 fully reduced ferredoxin;AQDS:蒽醌2, 6-二磺酸钠 Anthraquinone-2, 6-disulfonic Acid Disodium.
Fig. 3 Pathway proposed for Fe(III)-dependent methane oxidation and conservation of energy by M. acetivorans (Yan et al., 2018) 5CH4+32Fe(III)+13ADP+13HPO4=→CH3COOH+32Fe(II)+19H++13ATP +3CO2+5H2O ΔG°′= -2880.7 kJ
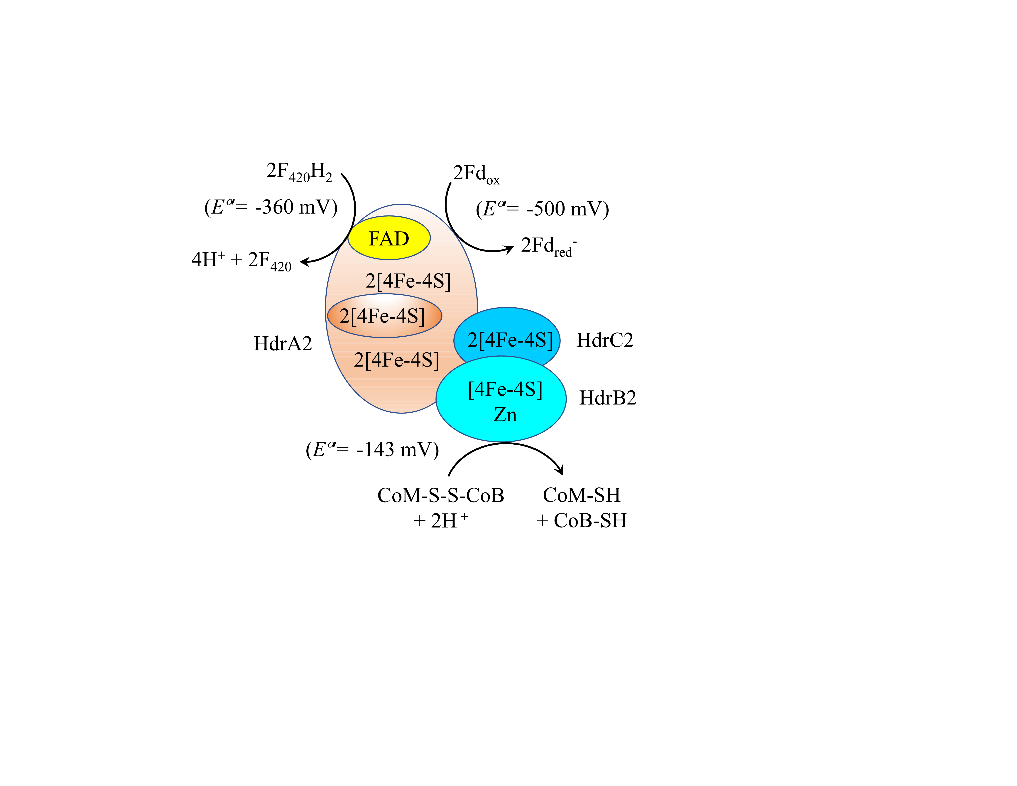
图4 胞质异二硫化物还原酶HdrA2B2C2利用CoM-S-S-CoB和F420H2还原Fdox假设机制(Yan et al.,2017) Fdred-:一个电子还原的铁氧化还原蛋白 one electron reduced ferredoxin;Fdox:氧化态铁氧化还原蛋白 oxidized ferredoxin;F420:氧化态辅酶F420 oxidized coenzyme F420;F420H2:还原态辅酶F420 reduced coenzyme F420;CoB-SH:辅酶B coenzyme B;CoM-SH:辅酶M coenzyme M;CoM-S-S-CoB:辅酶M和辅酶B的异二硫化物 Heterodisulfide of coenzyme M and coenzyme B;HdrA2B2C2:异质二硫化物还原酶亚单位A2、B2和C2 heterodisulfide reductase subunits A2, B2, and C2;FAD:黄素二核苷酸?avin adenine dinucleotide;Fe-S:铁硫簇 iron-sulfur cluster
Fig. 4 Proposed scheme for the reduction of Fdox with CoM-S-S-CoB and F420H2 by cytoplasmic electron confurcating heterodisulfide reductase HdrA2B2C2 complex (Yan et al., 2017)
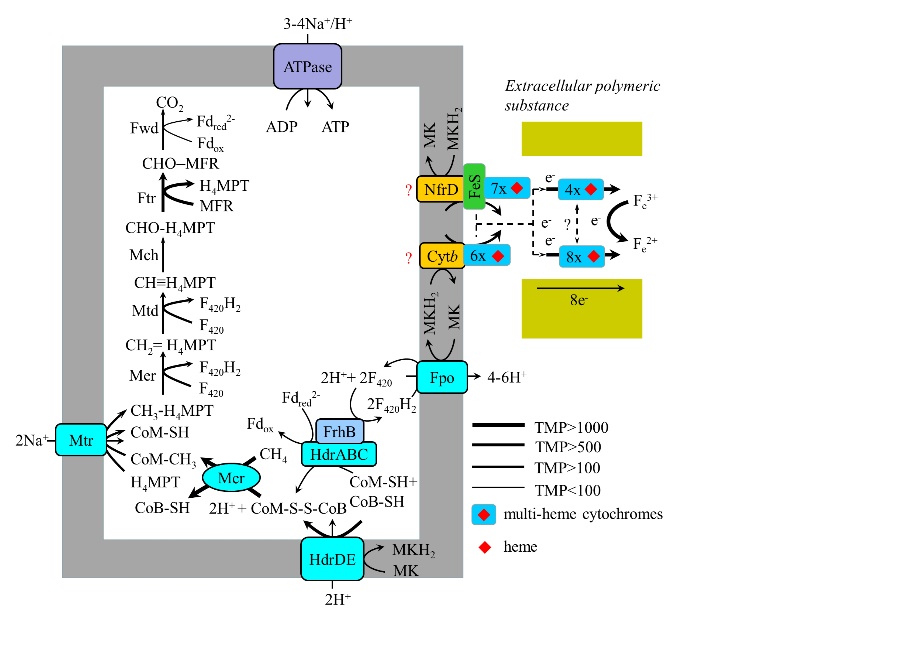
图5 M. ferrireducens Fe(Ⅲ)依赖的甲烷氧化及胞外电子传递假设途径(Cai et al.,2018) H4MPT:四氢甲烷蝶呤 tetrahydromethanopterin;MFR:甲烷呋喃 methanofuran;Fwd:甲酰甲烷呋喃脱氢酶 formyl-methanofuran dehydrogenase;Ftr:甲酰甲烷呋喃/四氢甲烷蝶呤甲酰转移酶 formylmethanofuran/H4MPT formyltransferase;Mch:甲基四氢甲烷蝶呤环化水解酶 methenyl-H4MPT cyclohydrolase;Mtd:辅酶F420依赖的亚甲基四氢甲烷蝶呤脱氢酶 F420-dependent methylene-H4MPT dehydrogenase;Mer:F420依赖的亚甲基四氢甲烷蝶呤还原酶 F420-dependent methylene-H4MPT reductase;Mtr:转运Na+的甲基四氢甲烷蝶呤:辅酶M甲基转移酶 Na+-translocating methyl-H4MPT:coenzyme M methyltransferase;Mcr:甲基辅酶M甲基还原酶 methyl-coenzyme M reductase;Fpo:还原态辅酶F420脱氢酶 F420H2 dehydrogenase;ATPase:腺苷三磷酸合成酶 ATP synthetase;HdrDE:异二硫化物还原酶亚单位D和E subunits D and E of heterodisulfide reductase;HdrABC:异二硫化物还原酶亚单位A、B和C2 subunits A, B, and C of heterodisulfide reductase;FrhB:辅酶F420还原氢化酶B亚单位 subunit B of F420-reducing hydrogenase;cytb:b型细胞色素 b-type cytochrome;NrfD:多硫化物还原酶D亚单位 subunit D of polysulfide reductase;FeS:铁氧还蛋白铁硫蛋白 ferredoxin iron sulfur protein;MK:氧化态甲基萘醌 oxidized menaquinone;MKH2:还原态甲基萘醌reduced menaquinone;F420H2:还原态辅酶F420 reduced coenzyme F420;F420:氧化态辅酶F420 oxidized coenzyme F420;CoB-SH:辅酶B coenzyme B;CoM-SH:辅酶M coenzyme M;CoM-S-S-CoB:辅酶M和辅酶B的异二硫化物 Heterodisulfide of coenzyme M and coenzyme B;Fdox:氧化态铁氧化还原蛋白 oxidized ferredoxin;Fdred2-:完全还原的铁氧化还原蛋白 fully reduced ferredoxin。下同 The same below
Fig. 5 Metabolic construction of the putative pathway for AOM coupled to Fe(III) reduction in “M. ferrireducens” (Cai et al., 2018)
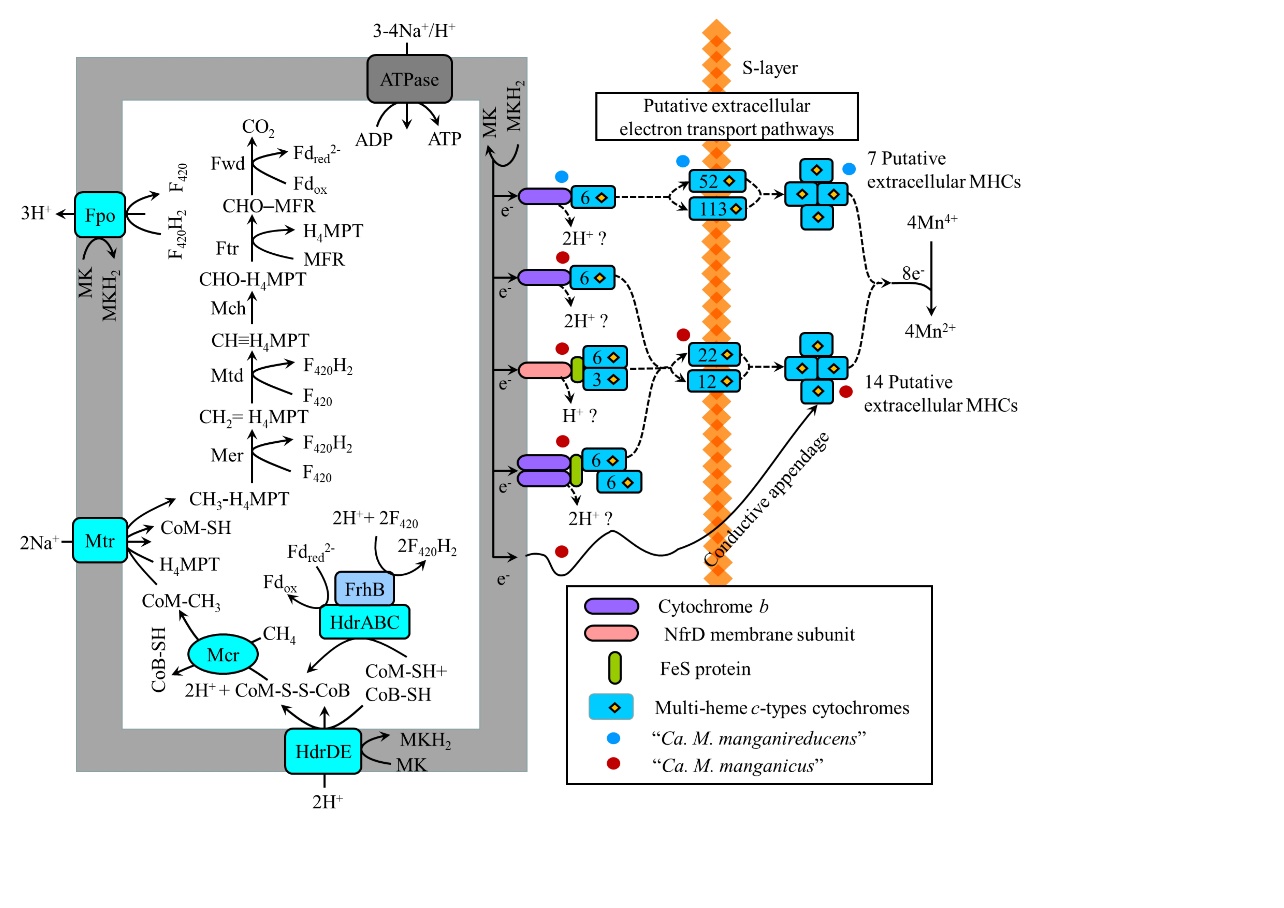
图6 Ca. Methanoperedens基因组中AOM与Mn(IV)还原偶联的代谢途径构建(Leu et al.,2020)
Fig. 6 Metabolic construction of the putative pathway for AOM coupled to Mn(IV) reduction in the “Ca. Methanoperedens” genomes (Leu et al., 2020)
| [1] |
AL-HASIN A, GURMAN S J, MURPHY L M, et al., 2010. Remediation of chromium(vi) by a methane-oxidizing bacterium[J]. Environmental Science & Technology, 44(1): 400-405.
DOI URL |
| [2] |
AMOS R T, BEKINS B A, COZZARELLI I, 2012. Evidence for iron- mediated a)anaerobic methane oxidation in a crude oil-contaminated aquifer[J]. Geobiology, 10(6): 506-517.
DOI URL |
| [1] | AROMOKEYE D A, KULKARNI A C, ELVERT M, et al., 2020. Rates and Microbial Players of Iron-Driven Anaerobic Oxidation of Methane in Methanic Marine Sediments[J]. Frontier in Microbiology, 10: 3041. |
| [2] |
BAR-OR I, ELVERT M, ECKERT W, et al., 2017. Iron-Coupled Anaerobic Oxidation of Methane Performed by a Mixed Bacterial-Archaeal Community Based on Poorly Reactive Minerals[J]. Environmental Science & Technology, 51(21): 12293-12301.
DOI URL |
| [3] |
BEAL E J, HOUSE C H, ORPHAN V J, 2009. Manganese- and iron-dependent marine methane oxidation[J]. Science, 325(5937): 184-187.
DOI URL |
| [4] |
CAI C, LEU A O, XIE G J, et al., 2018. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction[J]. ISME Journal, 12(8): 1929-1939.
DOI URL |
| [5] |
CALDWELL S L, LAIDLER J R, BREWER E A, et al., 2008. Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms[J]. Environmental Science & Technology, 42(18): 6791-6799.
DOI URL |
| [6] |
CHANG Y H, CHENG T W, LAI W J, et al., 2012. Microbial methane cycling in a terrestrial mud volcano in eastern Taiwan[J]. Environmental Microbiology, 14(4): 895-908.
DOI URL |
| [7] |
CROWE S, KATSEV S, LESLIE K, et al., 2011. The methane cycle in ferruginous Lake Matano[J]. Geobiology, 9(1): 61-78.
DOI URL |
| [8] |
CUI M M, MA A Z, QI H Y, et al., 2015. Anaerobic oxidation of methane: An “active” microbial process[J]. Microbiology Open, 4(1): 1-11.
DOI URL |
| [9] |
DONG Q Y, WANG Z, SHI L D, et al., 2019. Anaerobic methane oxidation coupled to chromate reduction in a methane-based membrane biofilm batch reactor[J]. Environmental Science and Pollution Research, 26(25): 26286-26292.
DOI URL |
| [10] |
EGGER M, KRAAL P, JILBERT T, et al., 2016. Anaerobic oxidation of methane alters diagenetic records of sulfur, iron and phosphorus in Black Sea sediments[J]. Biogeosciences, 13(18): 5333-5355.
DOI URL |
| [11] |
EGGER M, RASIGRAF O, SAPARt C J, et al., 2015. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments[J]. Environmental Science & Technology, 49(1): 277-283.
DOI URL |
| [12] |
ETTWIG K F, ZHU B, SPETH D, et al., 2016. Archaea catalyze iron-dependent anaerobic oxidation of methane[J]. PNAS, 113(45): 12792-12796.
DOI URL |
| [13] | I) Water Research, 88: 808-815. |
| [14] |
HANSEL C M, LENTINI C J, TANG Y Z, 2015. Dominance of sulfur-fueled iron oxide reduction in lowsulfate freshwater sediments[J]. ISME J, 9(11): 2400-2412.
DOI URL |
| [15] |
HE Q X, YU L P, LI J B, et al., 2019. Electron shuttles enhance anaerobic oxidation of methane coupled to iron (III) reduction[J]. Science of the Total Environment, 688: 664-672.
DOI URL |
| [16] |
HE Z F, ZHANG Q Y, FENG Y D, et al., 2018. Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane[J]. Science of the Total Environment, 610-611: 759-768.
DOI URL |
| [17] |
HOEHLER T M, ALPERIN M J, ALBERT D B, et al., 1994. Field and laboratory studies of methane oxidation in an anoxic marine sediment: evidence for a methanogen-sulfate reducer consortium[J]. Global Biogeochemical Cycles, 8(4): 451-463.
DOI URL |
| [18] |
KAMLAGE B, BLAUT M, 1992. Characterization of Cytochromes from Methanosarcina Strain Göl and Their Involvement in Electron Transport during Growth on Methanol[J]. Journal of Bacteriology, 174(12): 3921-3927.
DOI URL |
| [19] |
KISHI S, SAITO K, KATO Y, et al., 2017. Redox potentials of ubiquinone, menaquinone, phylloquinone, and plastoquinone in aqueous solution[J]. Photosynthesis Research, 134(2): 193-200.
DOI URL |
| [20] |
LAI C Y, DONG Q Y, CHEN J X, et al., 2018a. Role of extracellular polymeric substances in a methane based membrane biofilm reactor reducing vanadate[J]. Environmental Science & Technology, 52(18): 10680-10688.
DOI URL |
| [21] |
LAI C Y, DONG Q Y, RITTMANN B E, et al., 2018b. Bioreduction of antimonate by anaerobic methane oxidation in a membrane biofilm batch reactor[J]. Environmental Science & Technology, 52(15): 8693-8700.
DOI URL |
| [22] |
LAI C Y, WEN L L, SHI L D, et al., 2016. Selenate and nitrate bioreductions using methane as the electron donor in a membrane biofilm reactor[J]. Environmental Science & Technology, 50(18): 10179-10186.
DOI URL |
| [23] |
LEU A O, CAI C, MCILROY S J, et al., 2020. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae [J]. The ISME Journal, 14(4): 1030-1041.
DOI URL |
| [24] |
LIU J R, IZON G, WANG J S, et al., 2018. Vivianite formation in methane-rich deep-sea sediments from the South China Sea[J]. Biogeosciences, 15(20): 6329-6348.
DOI URL |
| [25] |
LOVLEY D R, 2012. Electromicrobiology[J]. Annual Review of Microbiology, 66: 391-409.
DOI URL |
| [26] |
LOVLEY D R, PHILLIPS E J P, LONERGAN D J, 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens[J]. Applied and Environmental Microbiology, 55(3): 700-706.
DOI URL |
| [27] |
LU Y Z, CHEN G J, BAI Y N, et al., 2018. Chromium isotope fractionation during Cr(VI) reduction in a methane-based hollow-fiber membrane biofilm reactor[J]. Water Research, 130: 263-270.
DOI URL |
| [28] |
LU Y Z, FU L, DING J, 2016. Cr(VI) reduction coupled with anaerobic oxidation of methane in a laboratory reactor[J]. Water Research, 102: 445-452.
DOI URL |
| [29] |
LUO J H, CHEN H, HU S H, et al., 2018. Microbial selenate reduction driven by a denitrifying anaerobic methane oxidation biofilm[J]. Environmental Science & Technology, 52(7): 4006-4012.
DOI URL |
| [30] |
LUO J H, WU M X, LIU J Y, et al., 2019. Microbial chromate reduction coupled with anaerobic oxidation of methane in a membrane biofilm reactor[J]. Environment International, 130: 104926.
DOI URL |
| [31] |
MCGLYNN S E, 2017. Energy metabolism during anaerobic methane oxidation in ANME archaea[J]. Microbes and Environments, 32(1): 5-13.
DOI URL |
| [32] | NORÐI K, THAMDRUP B, SCHUBERT C J, 2013. Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment[J]. Limnology & Oceanography, 58(2): 546-554. |
| [33] |
ONI O, MIYATAKE T, KASTEN S, 2015. Distinct microbial populations are tightly linked to the profile of dissolved iron in the methanic sediments of the Helgoland mud area, North Sea[J]. Frontiers in Microbiology, DOI: 10.3389/fmicb.2015.00365.
DOI |
| [34] |
PENG X T, GUO Z X, CHEN S, et al., 2017. Formation of carbonate pipes in the northern Okinawa Trough linked to strong sulfate exhaustion and iron supply[J]. Geochimica et Cosmochimica Acta, 205: 1-13.
DOI URL |
| [35] |
REEBURGH W S, 1976. Methane consumption in Cariaco Trench waters and sediments[J]. Earth and Planetary Science Letters, 28(3): 337-344.
DOI URL |
| [36] |
RIEDINGER N, FORMOLO M J, LYONS T W, 2014. An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments[J]. Geobiology, 12(2): 172-181.
DOI URL |
| [37] |
ROLAND F A E, BORGES A V, DARCHAMBEAU F, et al., 2021. The possible occurrence of iron-dependent anaerobic methane oxidation in an Archean Ocean analogue[J]. Scientific Reports, 11(1): 1597.
DOI URL |
| [38] | ROOZE J, EGGER M, TSANDEV I, et al., 2016. Iron-dependent anaerobic oxidation of methane in coastal surface sediments: Potential controls and impact[J]. Limnology & Oceanography, 61(S1): S267-S282. |
| [39] |
ROSENBAUM M, AULENTA F, VILLANO M, et al., 2011. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved[J]. Bioresource Technology, 102(1): 324-333.
DOI URL |
| [40] |
SCHELLER S, YU H, CHADWICK G L, et al., 2016. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction[J]. Science, 351(6274): 703-707.
DOI URL |
| [41] |
SEGARRA K E A, COMERFORD C, SLAUGHTER J, et al., 2013. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments[J]. Geochimica et Cosmochimica Acta, 115: 15-30.
DOI URL |
| [42] |
SHI L D, DU J J, WANG L B, et al., 2019. Formation of nanoscale Te0 and its effect on TeO32- reduction in CH4-based membrane biofilm reactor[J]. Science of the Total Environment, 655: 1232-1239.
DOI URL |
| [43] |
SHI L D, GUO T, LV P L, et al., 2020. Coupled anaerobic methane oxidation and reductive arsenic mobilization in wetland soils[J]. Nature Geoscience, 13(12): 799-805.
DOI URL |
| [44] | SIVAN O, ADLER M, PEARSON A, et al., 2011. Geochemical evidence for iron-mediated anaerobic oxidation of methane[J]. Limnology & Oceanography, 56(4): 1536-1544. |
| [45] |
SIVAN O, ANTLER G, TURCHYN A V, et al., 2014a. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps[J]. PNAS, 111(40): E4139-4147.
DOI URL |
| [46] |
SIVAN O, ANTLER G, TURCHYN A V, et al., 2014b. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps[J]. PNAS, 111(40): 4139-4147.
DOI URL |
| [47] |
SIVAN O, SCHRAG D P, MURRAY R W, 2007. Rates of methanogenesis and methanotrophy in deep-sea sediments[J]. Geobiology, 5(2): 141-151.
DOI URL |
| [48] |
SOO V W, MCANULTY M J, TRIPATHI A, et al., 2016. Reversing methanogenesis to capture methane for liquid biofuel precursors[J]. Microbial Cell Factories, DOI: 10.1186/s12934-015-0397-z.
DOI |
| [49] |
SU G Y, ZOPFI J, YAO H Y, et al., 2020. Manganese/iron-supported sulfate-dependent anaerobic oxidation of methane by archaea in lake sediments[J]. Limnology and Oceanography, 65(4): 863-875.
DOI URL |
| [50] |
TIETZE M, BEUCHLE A, LAMLA I, et al., 2003. Redox Potentials of Methanophenazine and CoB-S-S-CoM, Factors Involved in Electron Transport in Methanogenic Archaea[J]. Chembiochem, 4(4): 333-335.
DOI URL |
| [51] |
TIMMERS P H A, WELTE C U, KOEHORST J J, et al., 2017. Reverse Methanogenesis and Respiration in Methanotrophic Archaea[J]. Archaea-an International Microbiological Journal, DOI: 10.1155/2017/ 1654237.
DOI |
| [52] | TORRES N T, OCH L M, HAUSER P C, et al., 2014. Early diagenetic processes generate iron and manganese oxide layers in thesediments of Lake Baikal, Siberia[J]. Environmental Science Processes & Impacts, 16(4): 879-889. |
| [53] |
TREUDE T, KRAUSE S, MALTBY J, et al., 2014. Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implication for deep sulfur cycling[J]. Geochimica et Cosmochimica Acta, 144: 217-237.
DOI URL |
| [54] |
WEBER H S, HABICHT K S, THAMDRUP B, 2017. Anaerobic methanotrophic archaea of the ANME-2d cluster are active in a low-sulfate, iron-rich freshwater sediment[J]. Frontier in Microbiology, DOI: 10.3389/fmicb.2017.00619.
DOI |
| [55] |
WINKEL M, MITZSCHERLING J, OVERDUIN P P, et al., 2018. Anaerobic methanotrophic communities thrive in deep submarine permafrost[J]. Scientific reports, 8(1): 1291.
DOI URL |
| [56] |
WOLF M, KAPPLER A, JIANG J, et al., 2009. Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens[J]. Environmental Science & Technology, 43(15): 5679-5685.
DOI URL |
| [57] |
WU D D, XIE R, LIU J, et al., 2020. Zone of metal-driven anaerobic oxidation of methane is an important sink for phosphorus in the Taixinan Basin, South China Sea[J]. Marine Geology, 427: 106268.
DOI URL |
| [58] |
XIE R, WU D D, LIU J, et al., 2019. Geochemical Evidence of Metal-Driven Anaerobic Oxidation of Methane in the Shenhu Area, the South China Sea[J]. International Journal of Environmental Research and Public Health, 16(19): 3559.
DOI URL |
| [59] |
YAN Z, JOSHI P, GORSKI C A, et al., 2018. A biochemical framework for anaerobic oxidation of methane driven by Fe(III)-dependent respiration[J]. Nature Communications, 9(1): 1642.
DOI URL |
| [60] | YAN Z, WANG M Y, FERRY J G, 2017. A Ferredoxin- and F420H2-Dependent, Electron-Bifurcating, Heterodisulfide Reductase with Homologs in the Domains Bacteria and Archaea[J]. mBio, 8(1): e02285-16. |
| [61] |
ZHANG B G, JIANG Y F, ZUO K C, et al., 2020. Microbial vanadate and nitrate reductions coupled with anaerobic methane oxidation in groundwater[J]. Journal of Hazardous Materials, DOI: 10.1016/j. jhazmat.2019.121228.
DOI |
| [62] |
ZOU Y C, ZHANG S J, HUO L L, et al., 2018. Wetland saturation with introduced Fe(III) reduces total carbon emissions and promotes the sequestration of DOC[J]. Geoderma, 325: 141-151.
DOI URL |
| [63] | 陈颖, 2014. 厌氧甲烷氧化微生物代谢分子机制及其潜在参与矿物形成机理的研究[D]. 上海: 上海交通大学. |
| CHENG Y, 2014. Molecular metabolism study on microbial anaerobic methane oxidation and the associated biogenic minerals[D]. Shanghai: Shanghai Jiao Tong University. | |
| [64] | 何丹, 张尔翼, 余林鹏, 等, 2020. 微生物甲烷厌氧氧化耦合金属还原研究进展[J]. 应用与环境生物学报, 26(4): 844-856. |
| HE D, ZANG E Y, YU L P, et al., 2020. Advances in the anaerobic microbial oxidation of methane that is coupled with metal reduction[J]. Chinese Journal of Applied and Environmental Biology, 26(4): 844-856. | |
| [65] | 李玉芳, 2016. 锰矿石人工湿地中甲烷厌氧氧化及锰价态变化研究[D]. 重庆: 重庆大学. |
| LI Y F, 2016. A Thesis Submitted to Chongqing University in Partial Fulfillment of the Requirement for Professional Degree[D]. Chongqing: Chongqing University. | |
| [66] | 王维奇, 2014. 闽江河口湿地甲烷厌氧氧化及其机制研究[D]. 福建: 福建师范大学. |
| WANG W Q, 2014. Anaerobic oxidation of methane and its mechanism in the Minjiang River estuarine wetland[D]. Fujian: Fujian Normal University. | |
| [67] | 吴忆宁, 2019. 生活垃圾填埋场厌氧环境下甲烷氧化微生物富集培养和种群特征研究[D]. 江苏: 苏州科技大学. |
| WU Y N, 2019. Study of Enrichment and Population Characteristics of Methanotrophs in an Anaerobic Domestic Landfill Environment[D]. Suzhou: Suzhou University of Science and Technology. | |
| [68] | 张乃方, 徐陈超, 张凯航, 等, 2018. 水稻田中甲烷厌氧氧化及其对大气二氧化碳浓度升高的响应[C]// 中国生态学学会学术年会暨微生物生态学国际研讨会. 广州: 2018-11-09-2018-11-12: 153. |
| ZANG N F, XU C C, ZANG K H, et al., 2018. Anaerobic oxidation of methane and its response to elevated atmospheric carbon dioxide concentration in paddy fields[C]// Annual Conference and International Symposium on Microbial Ecology, Ecological Society of China. Guangzhou: 2018-11-09-2018-11-12: 153. | |
| [69] | 张梦竹, 李琳, 刘俊新, 2012. 硝酸盐和硫酸盐厌氧氧化甲烷途径及氧化菌群[J]. 微生物学通报, 39(5): 702-710. |
| ZHANG M Z, LI L, LIU J X, 2012. The parth way and methanotroph of anaerobic methane oxidation driven by nitrate or sulfate[J]. Microbiology China, 39(5): 702-710. |
| [1] | 刘紫薇, 葛继稳, 王月环, 杨诗雨, 姚东, 谢金林. 大九湖泥炭湿地甲烷通量变异特征及影响因素[J]. 生态环境学报, 2023, 32(4): 706-714. |
| [2] | 张涵, 唐常源, 禤映雪, 江涛, 黄品怡, 杨秋, 曹英杰. 珠江口红树林土壤甲烷和二氧化碳通量特征及其影响因素研究[J]. 生态环境学报, 2022, 31(5): 939-948. |
| [3] | 贺晓佳, 冯书华, 蒋明, 李明锐, 湛方栋, 李元, 何永美. UV-B辐射对水稻根际土壤活性有机碳转化和产甲烷潜力的影响[J]. 生态环境学报, 2022, 31(3): 556-564. |
| [4] | 蔡锡安, 黄娟, 吴彤, 刘菊秀, 蒋芬, 王森浩. 植物叶片排放甲烷的初步研究[J]. 生态环境学报, 2021, 30(9): 1842-1847. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
