生态环境学报 ›› 2023, Vol. 32 ›› Issue (11): 1933-1941.DOI: 10.16258/j.cnki.1674-5906.2023.11.004
周舒( ), 于冰洋, 杜柯龙, 林榆文, 冯能佳, 智丹*(
), 于冰洋, 杜柯龙, 林榆文, 冯能佳, 智丹*( )
)
收稿日期:2023-03-21
出版日期:2023-11-18
发布日期:2024-01-17
通讯作者:
*智丹。E-mail: zhidan@hunau.edu.cn作者简介:周舒(1998年生),女,硕士研究生,研究方向为水环境修复。E-mail: zs13272011674@163.com
基金资助:
ZHOU Shu( ), YU Bingyang, DU Kelong, LIN Yuwen, FENG Nengjia, ZHI Dan*(
), YU Bingyang, DU Kelong, LIN Yuwen, FENG Nengjia, ZHI Dan*( )
)
Received:2023-03-21
Online:2023-11-18
Published:2024-01-17
摘要:
三唑酮(TDF)等有机农药在生产和使用中存在环境风险,其难以被常规水处理工艺有效去除。电化学氧化技术应用于水中有机农药去除极具潜力,有机农药降解效率与电化学阳极材料性能有关。以Ti/RuO2-IrO2、Pt、Ti4O7电极为电化学阳极,开展电化学氧化技术降解和矿化水中TDF研究,比较和评估了不同类型阳极电化学降解TDF和溶液中TOC的效率;考察了电流密度、TDF初始浓度、溶液初始pH等反应参数对TDF电化学降解效率的影响;探究了TDF电化学降解路径及其降解产物毒性对溶液毒性的影响。结果表明:与Ti/RuO2-IrO2和Pt平板阳极相比,Ti/Ti4O7平板阳极对水中TDF和溶液总有机碳(TOC)去除效果较好;Ti4O7平板和膜阳极均对水中TDF的降解和矿化具有较高活性,TDF电化学降解效率和溶液TOC去除率可达94.5%-95.7%和72.5%-75.5%,达到相同TOC去除率时Ti4O7膜阳极较其平板阳极反应能耗低低50%左右;水中TDF电化学降解效率与电流密度、TDF初始浓度、溶液初始pH值等反应参数有关,TDF电化学降解效率随电流密度增大而增大、随TDF初始浓度和溶液初始pH的增大而减少;水中TDF(m/z=294.5)电化学降解生成A(m/z=224.5)和B(m/z=103),A(m/z=224.5)继续氧化生成了C(m/z=173.5)和D(m/z=86),这些中间产物可进一步氧化为二氧化碳、水等无机物;TDF、A、B、C和D对水生生物分别呈有毒性、无害性、无害性、有害性和无害性,有毒性的TDF逐渐降解为无毒性的降解副产物,可能是造成TDF溶液毒性随着反应时间逐渐下降的原因。该研究表明TDF可被Ti4O7阳极电化学氧化技术高效降解和矿化,为电化学氧化技术去除水中TDF等有机农药的研究与实践提供思路借鉴。
中图分类号:
周舒, 于冰洋, 杜柯龙, 林榆文, 冯能佳, 智丹. 电化学氧化降解水中三唑酮效能与反应路径[J]. 生态环境学报, 2023, 32(11): 1933-1941.
ZHOU Shu, YU Bingyang, DU Kelong, LIN Yuwen, FENG Nengjia, ZHI Dan. Electrochemical Oxidation of Triazolone in Water: Degradation Efficiency, Energy Consumption and Reaction Pathway[J]. Ecology and Environment, 2023, 32(11): 1933-1941.
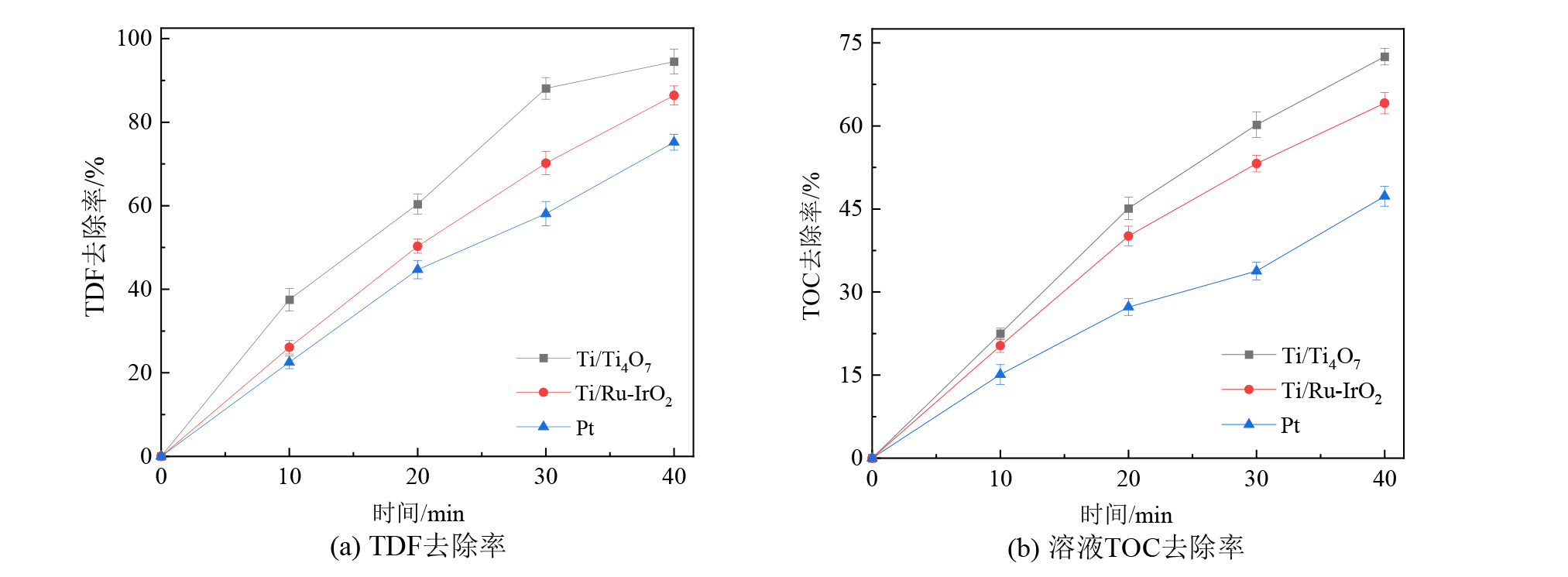
图2 Ti/Ti4O7、Ti/RuO2-IrO2和Pt平板阳极对水中TDF和TOC的去除率 电流密度15 mA?cm?2、极板间距10 mm、TDF初始浓度5 mg?L?1、30 mmol?L?1Na2SO4、pH为7.0
Figure 2 TDF and TOC removal efficiencies during TDF electro-oxidation by the Ti/Ti4O7, Ti/RuO2-IrO2 and Pt anodes
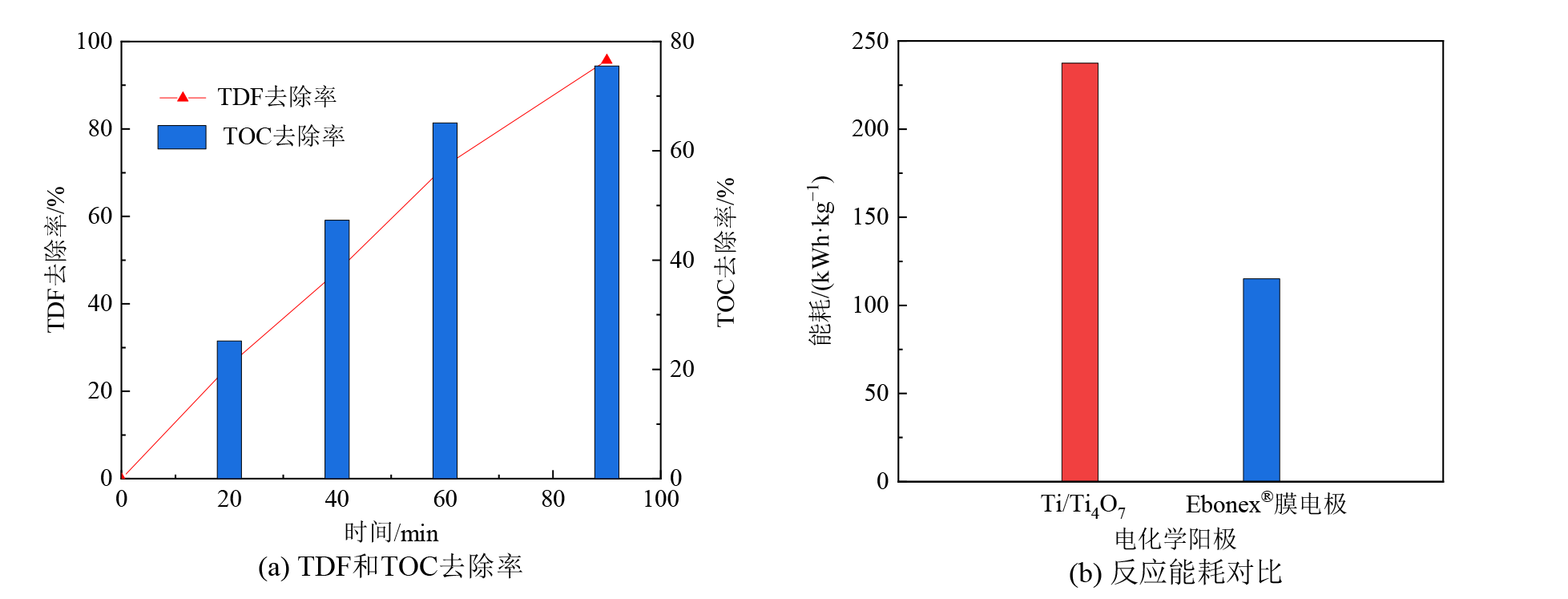
图4 Ebonex?膜阳极电化学降解TDF时的TDF去除率、TOC去除率和反应能耗
Figure 4 The TDF and TOC removal efficiencies, and the energy consumption of TDF electro-oxidation by the Ebonex? membrane anodes
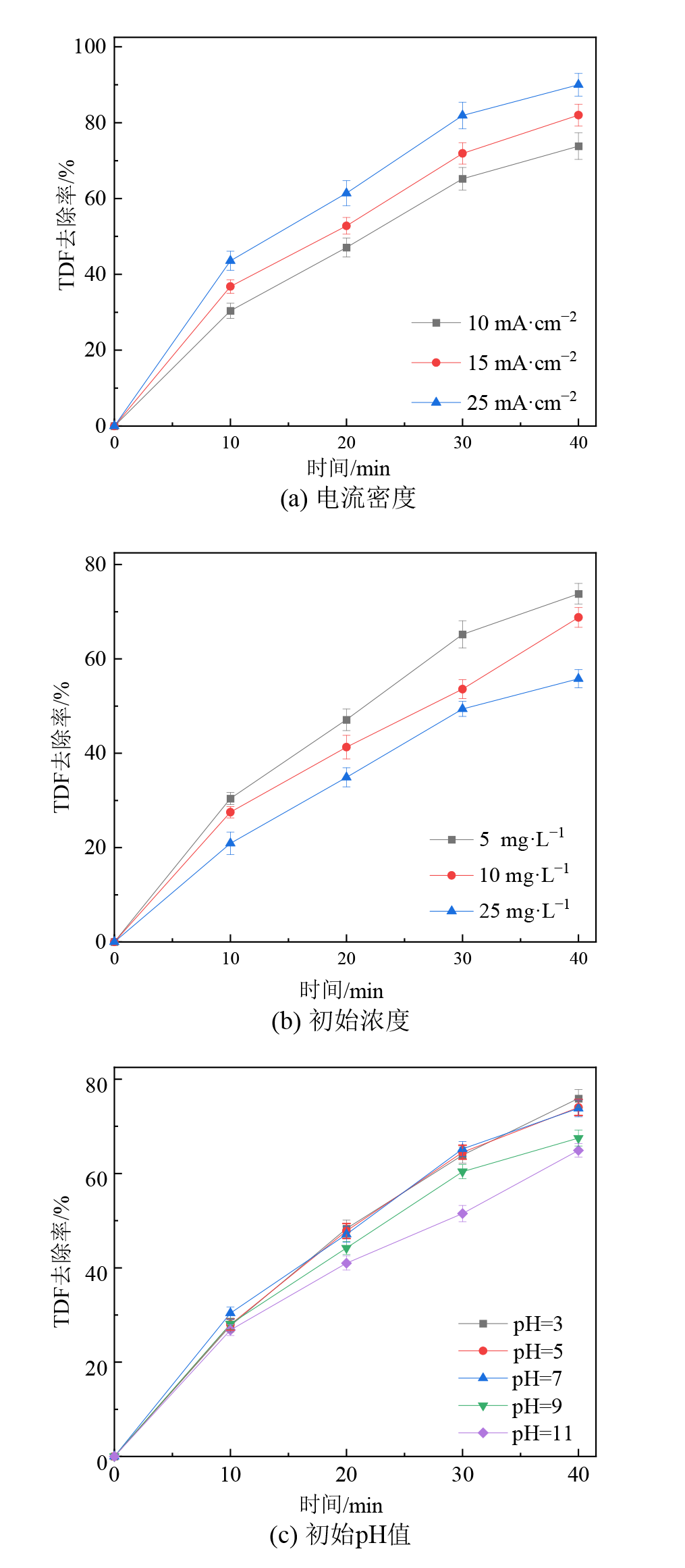
图5 不同电流密度、初始浓度和初始pH对TDF电化学降解效率的影响
Figure 5 Effects of the current densities, initial TDF concentration and initial solution pH on TDF degradation efficiency
| 化合物 | 质荷比 (m/z) | 分子式 | 产物离子质荷比 (m/z) | 结构 |
|---|---|---|---|---|
| TDF | 294.5 | C14H16ClN3O2 | 278, 243 | 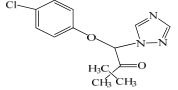 |
| A | 224.5 | C9H6ClN3O2 | 188 | 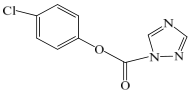 |
| B | 103 | C5H10O2 | 87, 85 | 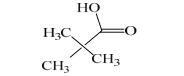 |
| C | 173.5 | C7H5ClO3 | 155, 137 | 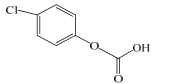 |
| D | 86 | C2H3N3O | 84, 68 | 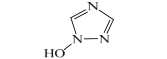 |
表1 HPLC-MS鉴别出的三唑酮及其降解中间产物
Table 1 Triazolones and their degradation intermediates identified by HPLC-MS
| 化合物 | 质荷比 (m/z) | 分子式 | 产物离子质荷比 (m/z) | 结构 |
|---|---|---|---|---|
| TDF | 294.5 | C14H16ClN3O2 | 278, 243 |  |
| A | 224.5 | C9H6ClN3O2 | 188 |  |
| B | 103 | C5H10O2 | 87, 85 |  |
| C | 173.5 | C7H5ClO3 | 155, 137 |  |
| D | 86 | C2H3N3O | 84, 68 |  |
| 物质 | 急性毒性/(mg∙L−1) | 慢性毒性(ChV)/(mg∙L−1) | 毒性 分类 | |||||
|---|---|---|---|---|---|---|---|---|
| 鱼类 (LC50) | 水蚤类 (LC50) | 藻类 (EC50) | 鱼类 (LC50) | 水蚤类 (LC50) | 藻类 (EC50) | |||
| TDF | 9.43b1) | 9.77b | 2.03b | 2.68b | 1.98b | 5.72b | 有毒性 | |
| A | 3510d3) | 1740d | 745d | 293d | 117d | 145d | 无害性 | |
| B | 2620d | 1410d | 854d | 241d | 120d | 200d | 无害性 | |
| C | 158d | 89.2c2) | 65.1c | 15.3c | 8.58b | 16.9c | 有害性 | |
| D | 57000d | 24000d | 5180d | 3910d | 1020d | 697d | 无害性 | |
表2 ECOSAR软件计算的三唑酮及其电化学降解中间产物的毒性值
Table 2 Toxicity values of triazolone and its electrochemical degradation intermediates calculated by ECOSAR software
| 物质 | 急性毒性/(mg∙L−1) | 慢性毒性(ChV)/(mg∙L−1) | 毒性 分类 | |||||
|---|---|---|---|---|---|---|---|---|
| 鱼类 (LC50) | 水蚤类 (LC50) | 藻类 (EC50) | 鱼类 (LC50) | 水蚤类 (LC50) | 藻类 (EC50) | |||
| TDF | 9.43b1) | 9.77b | 2.03b | 2.68b | 1.98b | 5.72b | 有毒性 | |
| A | 3510d3) | 1740d | 745d | 293d | 117d | 145d | 无害性 | |
| B | 2620d | 1410d | 854d | 241d | 120d | 200d | 无害性 | |
| C | 158d | 89.2c2) | 65.1c | 15.3c | 8.58b | 16.9c | 有害性 | |
| D | 57000d | 24000d | 5180d | 3910d | 1020d | 697d | 无害性 | |
| [1] |
BEJAN D, GUINEA E, BUNCE N J, 2012. On the nature of the hydroxyl radicals produced at boron-doped diamond and Ebonex® anodes[J]. Electrochimica Acta, 69: 275-281.
DOI URL |
| [2] |
CABEZA A, URTIAGA A M, ORTIZ I, 2007. Electrochemical treatment of landfill leachates using a boron-doped diamond anode[J]. Industrial & Engineering Chemistry Research, 46(5): 1439-1446.
DOI URL |
| [3] |
DOCEA A O, GOFITA E, GOUMENOU M, et al., 2018. Six months exposure to a real life mixture of 13 chemicals' below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats[J]. Food and Chemical Toxicology, 115: 470-481.
DOI PMID |
| [4] |
DUAN F, LI Y P, CAO H B, et al., 2015. Activated carbon electrodes: Electrochemical oxidation coupled with desalination for wastewater treatment[J]. Chemosphere, 125: 205-211.
DOI PMID |
| [5] |
GARCIA-MUNOZ P, DACHTLER W, ALTMAYER B, et al., 2020. Reaction pathways, kinetics and toxicity assessment during the photocatalytic degradation of glyphosate and myclobutanil pesticides: Influence of the aqueous matrix[J]. Chemical Engineering Journal, 384: 123315.
DOI URL |
| [6] |
GUO L, JING Y, CHAPLIN B P, 2016. Development and characterization of ultrafiltration TiO2 Magnéli phase reactive electrochemical membranes[J]. Environmental Science & Technology, 50(3): 1428-1436.
DOI URL |
| [7] |
HERMSEN S A B, BRANDHOF E-J V D, VAN DER VEN L T M, et al., 2011. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies[J]. Toxicology in Vitro, 25(3): 745-753.
DOI PMID |
| [8] |
LIANG S T, LIN H, YAN X F, et al., 2018. Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products and toxicity[J]. Chemical Engineering Journal, 332: 628-636.
DOI URL |
| [9] |
NAYAK S, CHAPLIN B P, 2018. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes[J]. Electrochimica Acta, 263: 299-310.
DOI URL |
| [10] |
SAMET Y, AGENGUI L, ABDEHEDI R, 2010. Electrochemical degradation of chlorpyrifos pesticide in aqueous solutions by anodic oxidation at boron-doped diamond electrodes[J]. Chemical Engineering Journal, 161(1-2): 167-172.
DOI URL |
| [11] |
SANTOS C M, ElABD Y A, JING Y, 2016. Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying[J]. Aiche Journal, 62(2): 508-524.
DOI URL |
| [12] |
TRELLU C, CHAPLIN B P, COETSIER C, et al., 2018. Electro-oxidation of organic pollutants by reactive electrochemical membranes[J]. Chemosphere, 208: 159-175.
DOI PMID |
| [13] | WATSCHKE T L, MUMMA R O, LINDE D T, et al., 1999. Surface runoff of selected pesticides applied to turfgrasses[J]. ACS Symposium Series, 743: 94-105. |
| [14] |
WANG J B, ZHI D, HAO Z, et al., 2018. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode[J]. Water Research, 137: 324-334.
DOI URL |
| [15] |
WANG G R, LIU Y, YE J W, et al., 2020. Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7anode[J]. Chemosphere, 241: 125084.
DOI URL |
| [16] |
YOU S J, LIU B, GAO Y F, et al., 2016. Monolithic porous Magnéli-phase Ti4O7 for electro-oxidation treatment of industrial wastewater[J]. Electrochimica Acta, 214: 326-335.
DOI URL |
| [17] |
YANG Y, HOFFMANN M R, 2016. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment[J]. Environmental Science & Technology, 50(21): 11888-11894.
DOI URL |
| [18] |
ZAKY A M, CHAPLIN B P, 2014. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane[J]. Environmental Science Technology, 48(10): 5857-5867.
DOI URL |
| [19] |
ZHI D, ZHANG J, WANG J B, et al., 2020a. Electrochemical treatments of coking wastewater and coal gasification wastewater with Ti/Ti4O7 and Ti/RuO2-IrO2 anodes[J]. Journal of Environmental Management, 265: 110571.
DOI URL |
| [20] |
ZHI D, WANG J, ZHOU Y, et al., 2020b. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction[J]. Chemical Engineering Journal, 383: 123149.
DOI URL |
| [21] |
ZHANG J, ZHOU Y Y, YAO B, et al., 2021. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique[J]. Journal of Hazardous Materials, 418: 126313.
DOI URL |
| [22] | 耿榕, 赵国华, 刘梅川, 等, 2010. 掺硼金刚石膜电极表面产生羟基自由基的原位ESR研究[J]. 物理化学学报, 26(6): 1493-1498. |
|
GENG R, ZHAO G H, LIU M C, et al., 2010. In situ ESR study of hydroxyl radical generation on a boron doped diamond film electrode surface[J]. Acta Physico-Chimica Sinica, 26(6): 1493-1498.
DOI URL |
|
| [23] | 黄礼丽, 何平, 2019. Ti/IrO2-RuO2电极降解苯酚红废水[J]. 云南化工, 46(1): 116-118. |
| HUANG L L, HE P, 2019. Degradation of phenol red wastewater by Ti/IrO2-RuO2 electrode[J]. Yunnan Chemical Technology, 46(1): 116-118. | |
| [24] |
何森华, 张起源, 郭洁, 等, 2021. 湛江红树林湿地沉积物中多溴联苯醚 (PBDEs) 的污染特征与生态风险评价[J]. 生态环境学报, 30(2): 368-375.
DOI |
| HE S H, ZHANG Q Y, GUO J, et al., 2010. Pollution characteristics and ecological risk assessment of polybrominated diphenyl ethers (PBDEs) in sediments of Zhanjiang Mangrove Wetland[J]. Ecology and Environmental Sciences, 30(2): 368-375. | |
| [25] | 刘娜, 金小伟, 穆云松, 等, 2017. 三唑酮在水环境中的环境行为、毒性效应及生态风险[J]. 生态毒理学报, 12(4): 65-75. |
| LIU N, JIN X W, MU Y S, et al., 2017. Review of environmental behavior, toxicity and ecological risk of triadimefon in the aquatic environment[J]. Asian Journal of Ecotoxicology, 12(4): 65-75. | |
| [26] | 李绍峰, 石冶, 崔崇威, 2008. 臭氧氧化降解三唑酮的试验[J]. 环境科学学报, 28(7): 1381-1388. |
| LI S F, SHI Y, CUI C W, 2008. Ozonation of endocrine disruptors (EDs) triadimefon in water[J]. Acta Scientiae Circumstantiae, 28(7): 1381-1388. | |
| [27] | 刘少颖, 2011. 三唑酮对斑马鱼的胚胎发育和内分泌-生殖毒性[D]. 杭州: 浙江大学:42-46. |
| LIU S Y, 2011. Embryonic developmental and endocrine-reproductive toxicity of triadimefon on zebrafish[D]. Hangzhou: Zhejiang University:42-46. | |
| [28] | 李慧媛, 高丁, 史江红, 等, 2017. Ti/RuO2-IrO2电极电化学方法降解溶液中TBBPA及其降解机理探究[J]. 环境科学学报, 37(2): 642-650. |
| LI H Y, GAO D, SHI J H, et al., 2017. Electrochemical degradation of tetrabromobisphenol A (TBBPA) in aqueous solutions by Ti /RuO2-IrO2 anode[J]. Acta Scientiae Circumstantiae, 37(2): 642-650. | |
| [29] | 卢强, 安立超, 钟秦, 等, 2010. 钛基锡锑电极电催化氧化处理硝基苯废水[J]. 化工环保, 30(2): 100-103. |
| LU Q, AN L C, ZHONG Q, et al., 2010. Treatment of nitrobenzene wastewater by electrocatalytic oxidation with Sn-Sb/Ti electrode[J]. Environmental Protection of Chemical Industry, 30(2): 100-103. | |
| [30] | 刘毅华, 2005. 三唑酮的水环境化学行为研究[D]. 长沙: 湖南农业大学: 1. |
| LIU Y H, 2005. Chemistry behavior of triadimefon in aquatic environment[D]. Changsha: Hunan Agricultural University: 1. | |
| [31] | 魏琛, 宋丽婧, 杨卫萍, 等, 2016. 贵阳市饮用水源地有机氯农药污染的检测与特征分析[J]. 环境科学与技术, 39(3): 131-135. |
| WEI C, SONG L J, YANG W P, et al., 2016. Detection and feature analysis of organochlorine pesticide pollution in drinking water sources in Guiyang[J]. Environmental Science & Technology, 39(3): 131-135. | |
| [32] | 吴震峰, 2012. 电化学高级氧化去除水环境中痕量六六六[D]. 济南: 山东大学: 21-25. |
| WU Z F, 2012. Degradation of trace BHC with electrochemical oxidation in the water environment[D]. Ji’nan: Shandong University: 21-25. | |
| [33] |
王茜, 王金龙, 唐小斌, 等, 2022. 某市水源水及净水厂中药品和个人护理品 (PPCPs) 的分布、含量和去除规律[J]. 生态环境学报, 31(6): 1193-1199.
DOI |
| WANG Q, WANG J L, TANG X B, et al., 2022. Concentration, distribution and fate of pharmaceuticals and personal care products (PPCPs) for drinking water systems in a city[J]. Ecology and Environmental Sciences, 31(6): 1193-1199. | |
| [34] | 张明贤, 魏琛, 盛贵尚, 等, 2019. 三维电极系统电化学氧化三唑酮[J]. 广州化学, 44(2): 32-38. |
| ZHANG M X, WEI C, SHENG G S, et al., 2019. Electro-chemical oxidation of aqueous triadimefon by three-dimensional electrode system[J]. Guangzhou Chemistry, 44(2): 32-38. | |
| [35] | 智丹, 王建兵, 王维一, 等, 2018a. Ti/Ti4O7阳极电化学氧化降解水中的美托洛尔[J]. 环境科学学报, 38(5): 1858-1867. |
| ZHI D, WANG J B, WANG W Y, et al., 2018. Electrochemical degradation of metoprolol in aquatic environment over a Ti/Ti4O7anode[J]. Acta Scientiae Circumstantiae, 38(5): 1858-1867. | |
| [36] | 智丹, 王建兵, 周云惠, 等, 2018b. 钛基锡锑阳极电化学氧化去除水中的四环素[J]. 环境工程学报, 12(1): 57-64. |
| ZHI D, WANG J B, ZHOU Y H, et al., 2018. Electrochemical oxidation of tetracycline in aquatic environment by Ti/SnO2-Sb anode[J]. Chinese Journal of Environmental Engineering, 12(1): 57-64. | |
| [37] | 智丹, 2018. 臭氧氧化复合电化学活性膜去除水中四环素的研究[D]. 北京: 中国矿业大学 (北京): 9. |
| ZHI D, 2018. Study on the removal of tetracycline by the reactive electrochemical membrane coupled with ozonation[D]. Beijing: China University of Mining and Technology (Beijing): 9. | |
| [38] | 中华人民共和国国家环境保护总局, 中国国家标准化管理委员会, 1995. 水质急性毒性的测定-发光细菌法: GB/T 15441—1995 [S]. 北京: 中国标准出版社: 618-623. |
| Ministry of Ecology and Environment of the People’s Republic of China, Standardization Administration, 1995. Water quality- Determination of the acute toxicity-Luminescent bacteria test: GB/T15441—1995 [S]. Beijing: Standards Press of China: 618-623. |
| [1] | 黄世聪, 陈丽珂, 张政杰, 陈科华, 陈澄宇, 曾巧云. 四环素对不同品种蔬菜毒性阈值及其敏感性分布[J]. 生态环境学报, 2023, 32(11): 1988-1995. |
| [2] | 刘安, 吴昊, 何贝贝. 陆地环境中纳米塑料毒性效应的研究进展[J]. 生态环境学报, 2023, 32(11): 2030-2040. |
| [3] | 苏焱, 全妍红, 宦紫嫣, 姚佳, 苏小娟. 磷改性生物炭对云南某铅锌矿周边农田铅锌污染土壤修复效果的影响[J]. 生态环境学报, 2022, 31(3): 593-602. |
| [4] | 刘沙沙, 陈诺, 杨晓茵. 微塑料对有机污染物的吸附-解吸特性及其复合毒性效应研究进展[J]. 生态环境学报, 2022, 31(3): 610-620. |
| [5] | 李涛, 孟丹丹, 郭水良, 袁国徽, 钱振官, 吕卫光. 17种常用除草剂对蚯蚓的急性毒性[J]. 生态环境学报, 2021, 30(6): 1269-1275. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
