生态环境学报 ›› 2023, Vol. 32 ›› Issue (10): 1822-1832.DOI: 10.16258/j.cnki.1674-5906.2023.10.011
李龙飞1,2( ), 魏颖1,2, 赵建南1,2, 董静1,2, 张景晓1,2, 高肖飞1,2, 张曼1,2, 袁华涛1,2, 高云霓1,2,*(
), 魏颖1,2, 赵建南1,2, 董静1,2, 张景晓1,2, 高肖飞1,2, 张曼1,2, 袁华涛1,2, 高云霓1,2,*( ), 李学军1,2
), 李学军1,2
收稿日期:2023-05-12
出版日期:2023-10-18
发布日期:2024-01-16
通讯作者:
*高云霓。E-mail: gaoyn@htu.cn作者简介:李龙飞(1998年生),男,硕士研究生,研究方向为水域生态学。E-mail: llf619@outlook.com
基金资助:
LI Longfei1,2( ), WEI Ying1,2, ZHAO Jiannan1,2, DONG Jing1,2, ZHANG Jingxiao1,2, GAO Xiaofei1,2, ZHANG Man1,2, YUAN Huatao1,2, GAO Yunni1,2,*(
), WEI Ying1,2, ZHAO Jiannan1,2, DONG Jing1,2, ZHANG Jingxiao1,2, GAO Xiaofei1,2, ZHANG Man1,2, YUAN Huatao1,2, GAO Yunni1,2,*( ), LI Xuejun1,2
), LI Xuejun1,2
Received:2023-05-12
Online:2023-10-18
Published:2024-01-16
摘要:
富营养水体微囊藻(Microcystis)等有害蓝藻生态防控的长效性与所处微生态系统的响应和影响有关,其中周丛藻类与沉水植物处于同一生态位,在淡水生态系统稳态转换方面具有重要作用。沉水植物对微囊藻等蓝藻的抑制作用研究较多,但周丛藻类如何响应和影响这一过程还不清楚。为此,选择3种常见水鳖科沉水植物苦草(Vallisneria natans)、轮叶黑藻(Hydrilla verticillata)、伊乐藻(Elodea nuttallii)健康植株与2株微囊藻(Microcystis sp.),在室内可控条件下分别共培养18 d,测定每株微囊藻和每种植物生长变化的同时,观察各实验组周丛藻类群落结构的响应。结果表明,鲜质量为2.0 g∙L−1的3种沉水植物对起始密度为 (3.5±0.1)×106 cells∙mL−1的2株微囊藻抑制作用显著,第6天,抑制率均超过80%,伊乐藻的抑藻效果最强。但3种植物也受到微囊藻不同程度的影响,苦草部分叶片从第6天开始死亡分解,伊乐藻和轮叶黑藻鲜质量和株长未明显增加。伴随着3种植物对微囊藻的有效抑制,水环境中氨氮浓度不断增加,在第9-12天达到峰值后再逐渐降低。从第9天开始,各实验组烧杯底部和内壁开始出现附着藻类。实验结束时,相比于植物单培对照组,植物与微囊藻共培组中周丛藻类密度更高,多样性更低。伊乐藻、轮叶黑藻与微囊藻共培组周丛蓝藻相对密度明显高于植物单培组,而苦草、轮叶黑藻与微囊藻共培组丝状蓝藻泽丝藻(Limnothrix sp.)、细鞘丝藻(Leptolyngbya sp.)相对密度高于植物单培组。由此推测,周丛藻类可吸收利用微囊藻死亡分解后释放的营养物质,降低水体营养水平,与沉水植物协同维持清水稳态,但周丛藻类中优势度较高的丝状蓝藻存在潜在生态风险,应加强监测与防控。
中图分类号:
李龙飞, 魏颖, 赵建南, 董静, 张景晓, 高肖飞, 张曼, 袁华涛, 高云霓, 李学军. 3种沉水植物对微囊藻的抑制作用及其周丛藻类响应[J]. 生态环境学报, 2023, 32(10): 1822-1832.
LI Longfei, WEI Ying, ZHAO Jiannan, DONG Jing, ZHANG Jingxiao, GAO Xiaofei, ZHANG Man, YUAN Huatao, GAO Yunni, LI Xuejun. The Inhibition of Microcystis by the Three Submerged Hydrocharitaceae Species and the Response of Periphytic Algae[J]. Ecology and Environment, 2023, 32(10): 1822-1832.
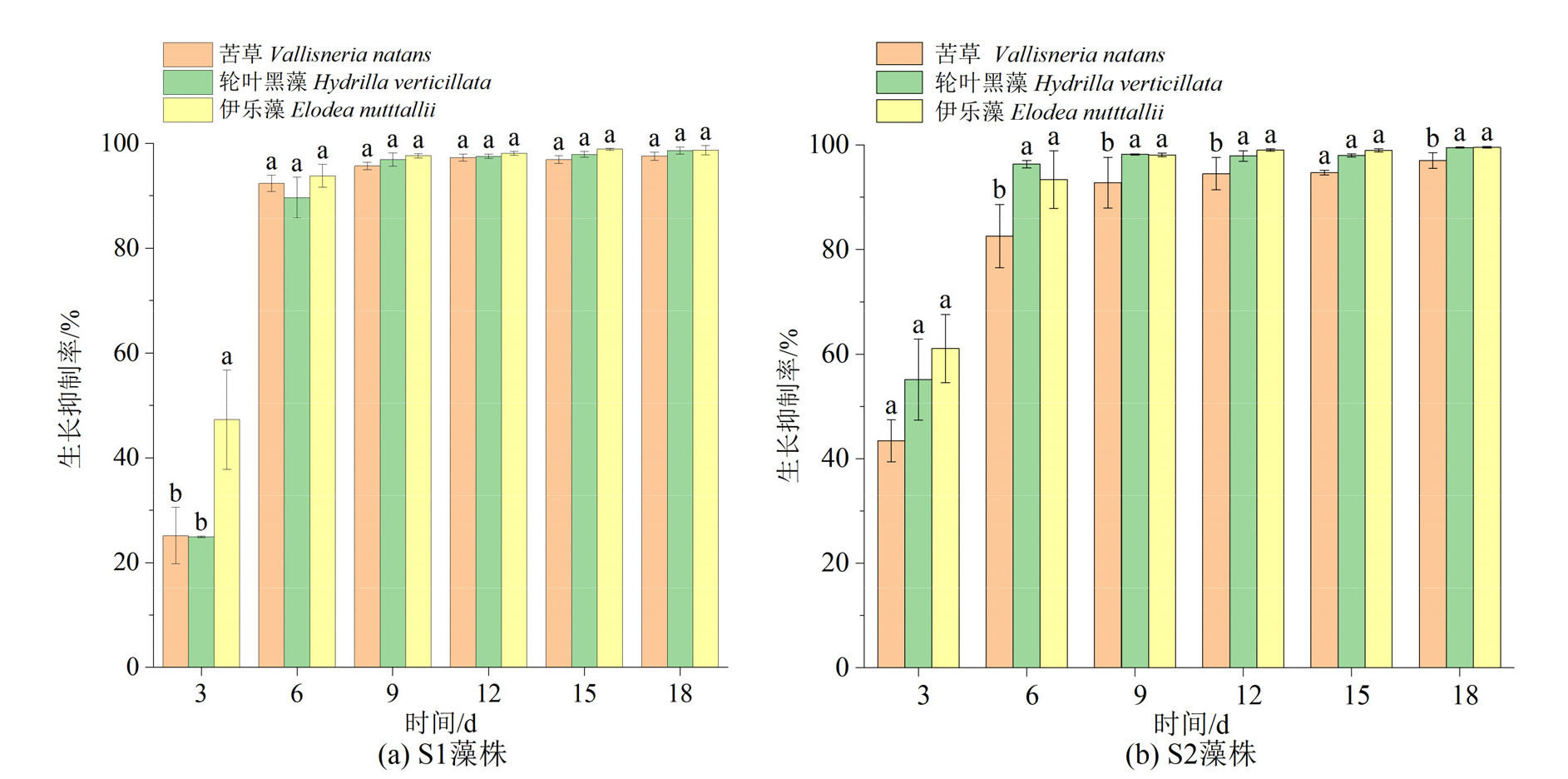
图1 苦草、轮叶黑藻和伊乐藻对2株微囊藻的生长抑制率 不同小写字母表示3种植物间的差异显著性(P<0.05)。下同
Figure 1 Growth inhibition rate of two strains of Microcystis by V. natans, H. verticillata and E. nutttallii
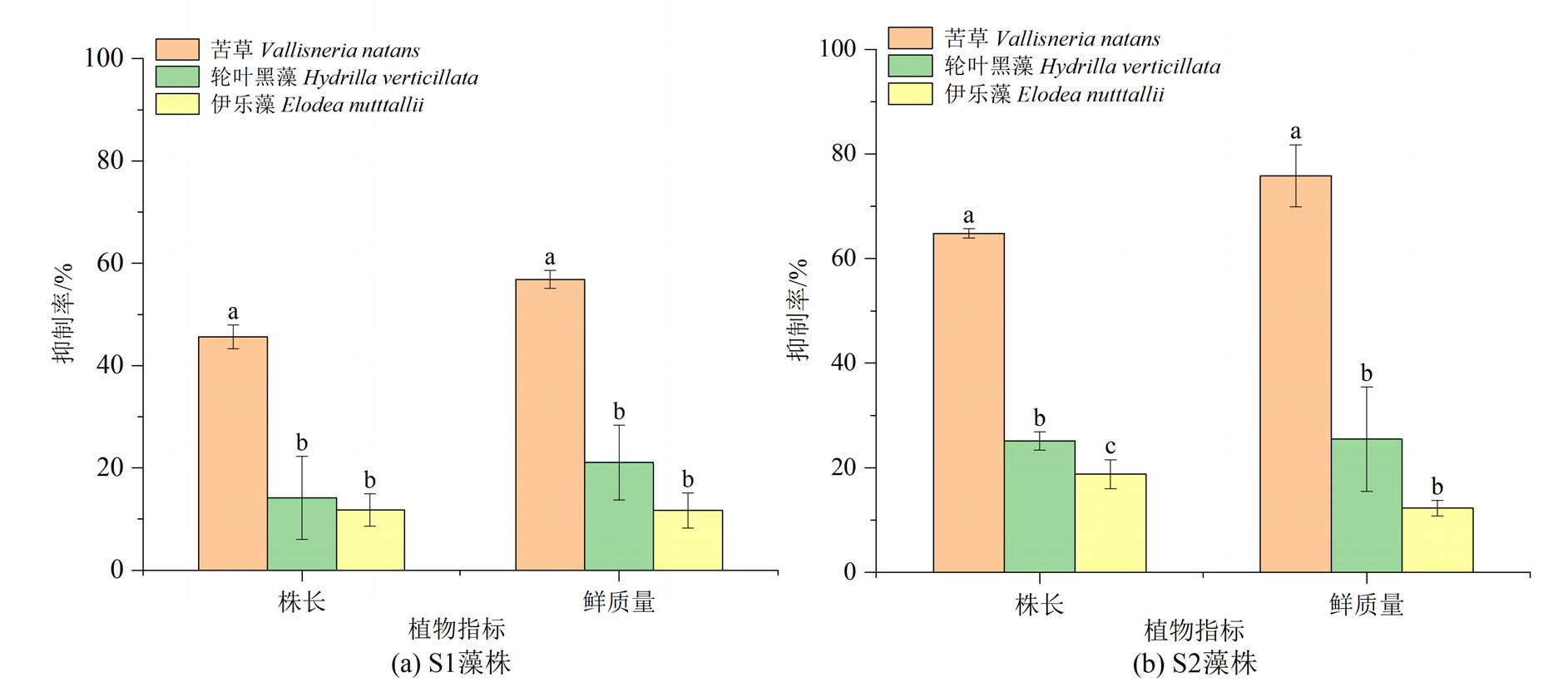
图2 2株微囊藻对苦草、轮叶黑藻和伊乐藻株长和鲜质量抑制率
Figure 2 Inhibition rate on length and fresh weight of V. natans, H. verticillata and E. nutttallii by two strains of Microcystis
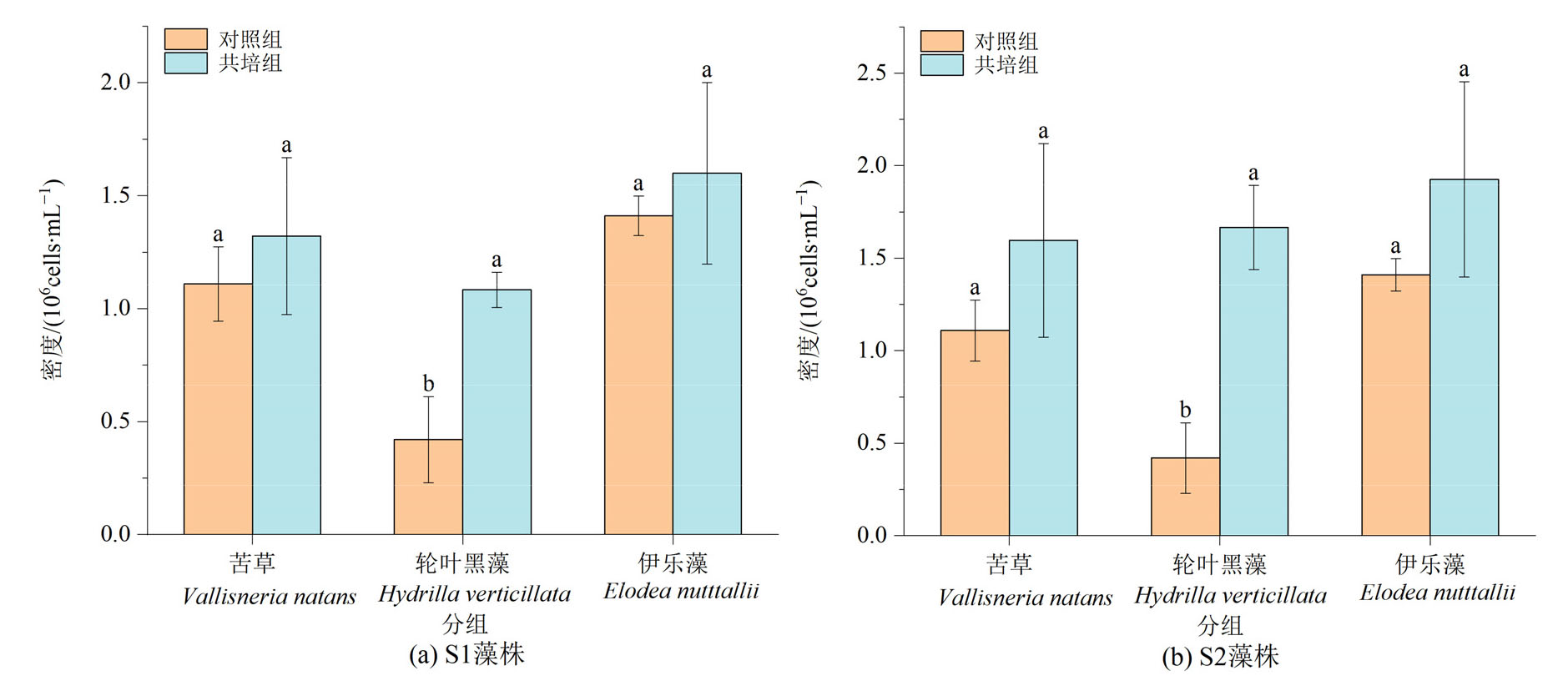
图3 实验结束时苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中周丛藻类密度 不同小写字母表示3种植物对照组与共培组的差异显著性(P<0.05)
Figure 3 Density of periphytic algae in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
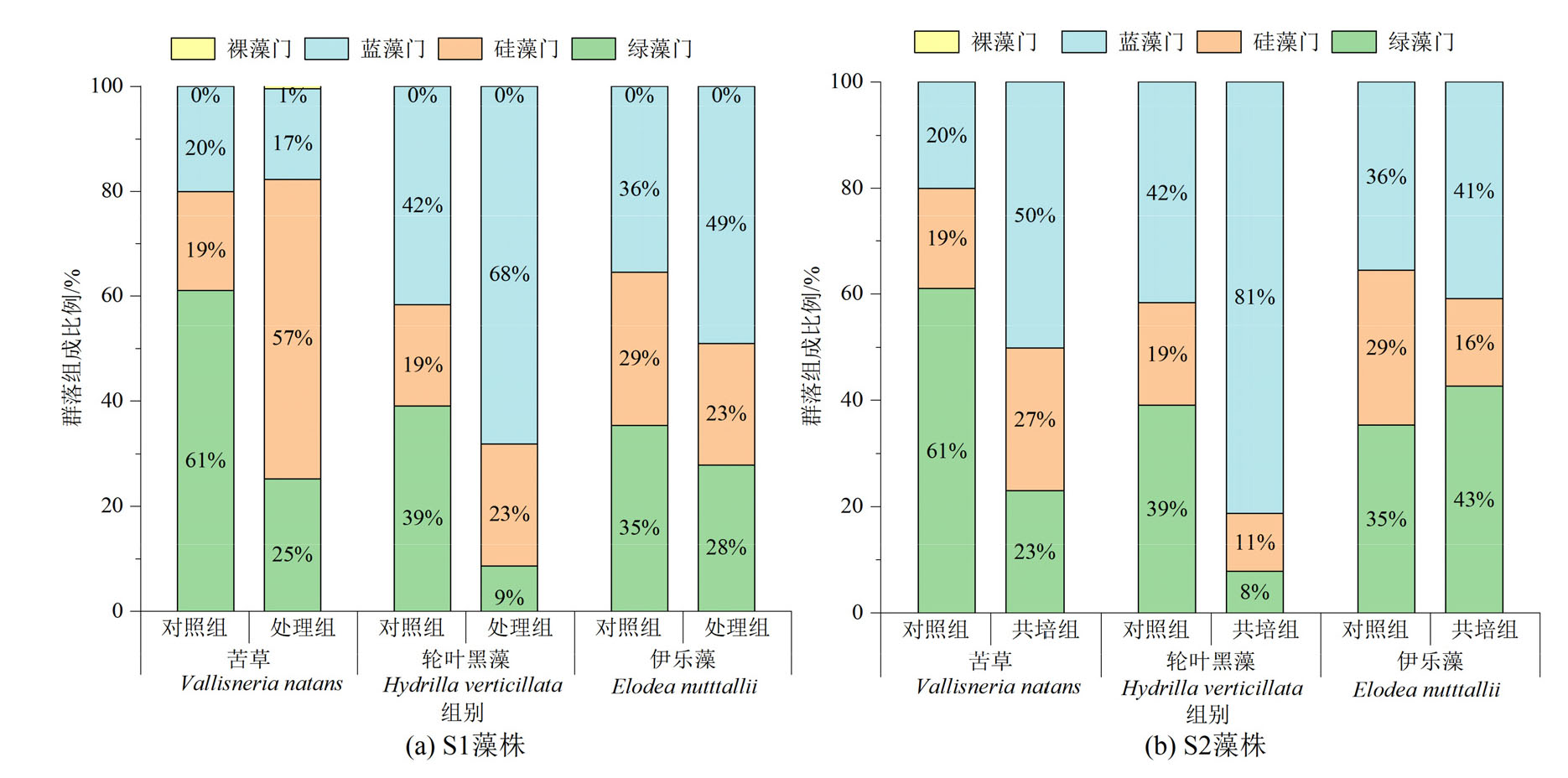
图4 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中周丛藻类门水平群落组成
Figure 4 Community composition of periphytic algae at phylum level in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
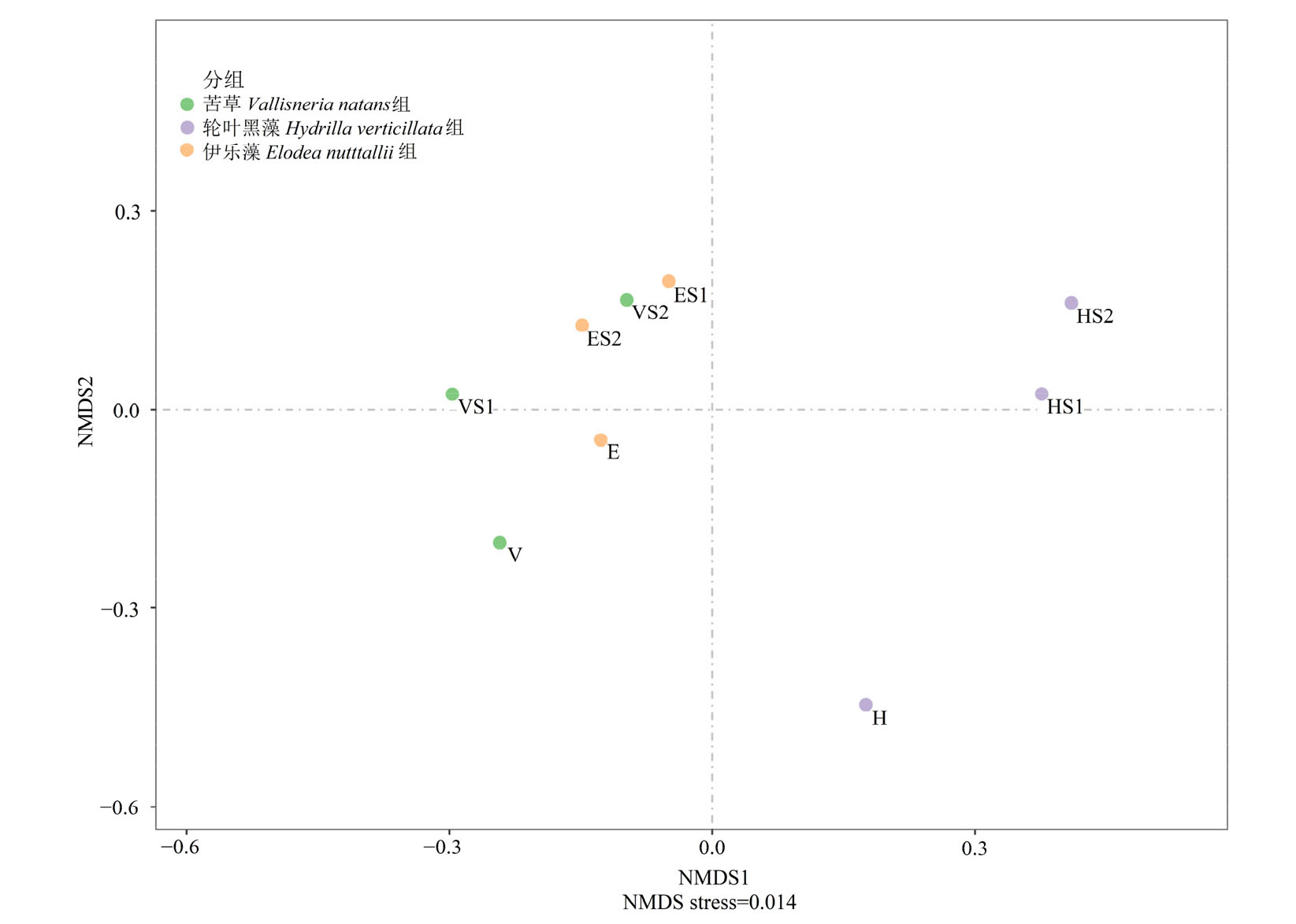
图5 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中周丛藻类NMDS分析 图中E、H、V代表伊乐藻、轮叶黑藻、苦草的对照组,ES1、HS1、VS1和ES2、HS2、VS2代表苦草、轮叶黑藻、伊乐藻与S1、S2藻株的共培组
Figure 5 Non-metric multidimensional scaling (NMDS) diagram of periphytic algae in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
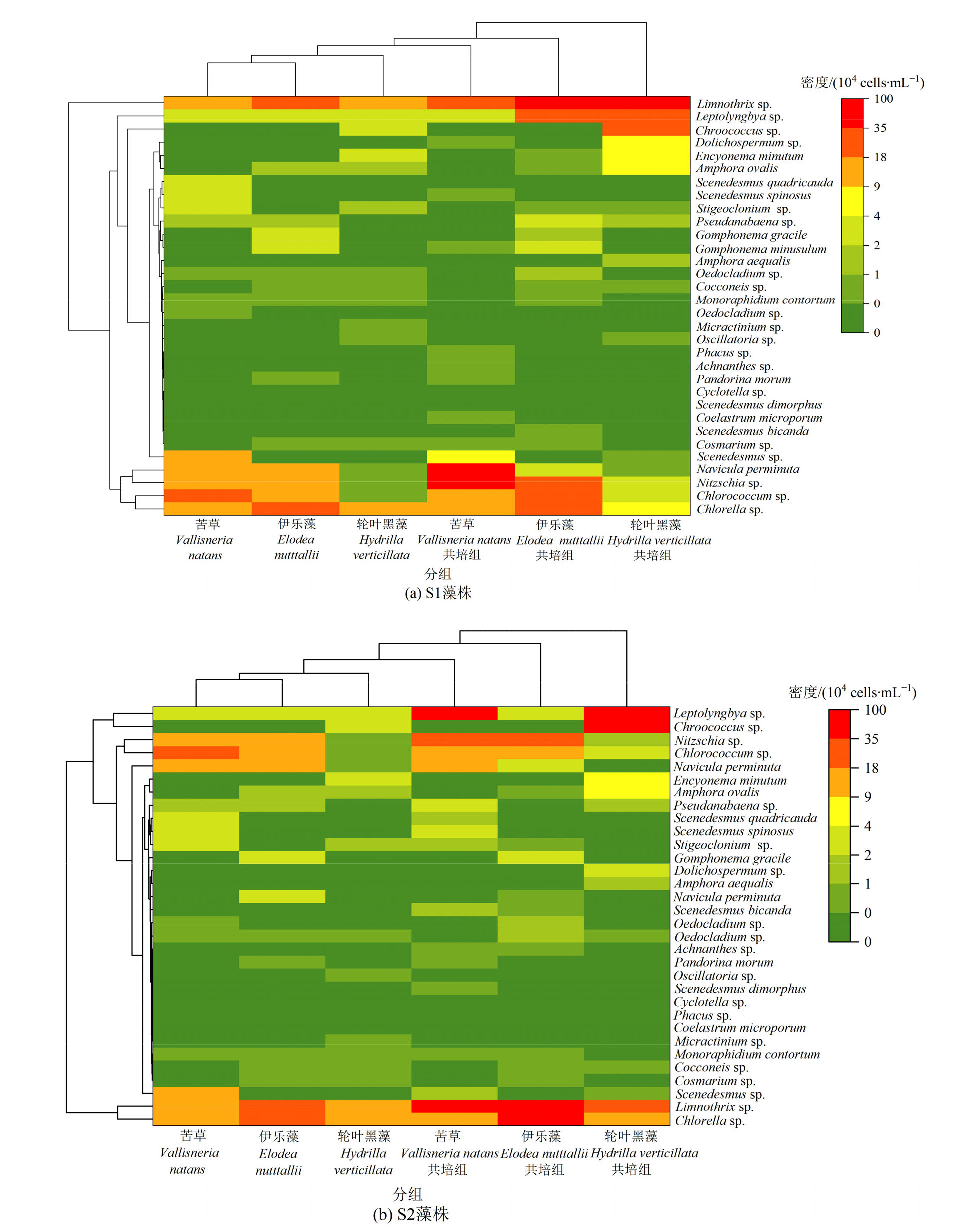
图6 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中周丛藻类属(种)水平群落组成
Figure 6 Community composition of periphytic algae at genus level in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
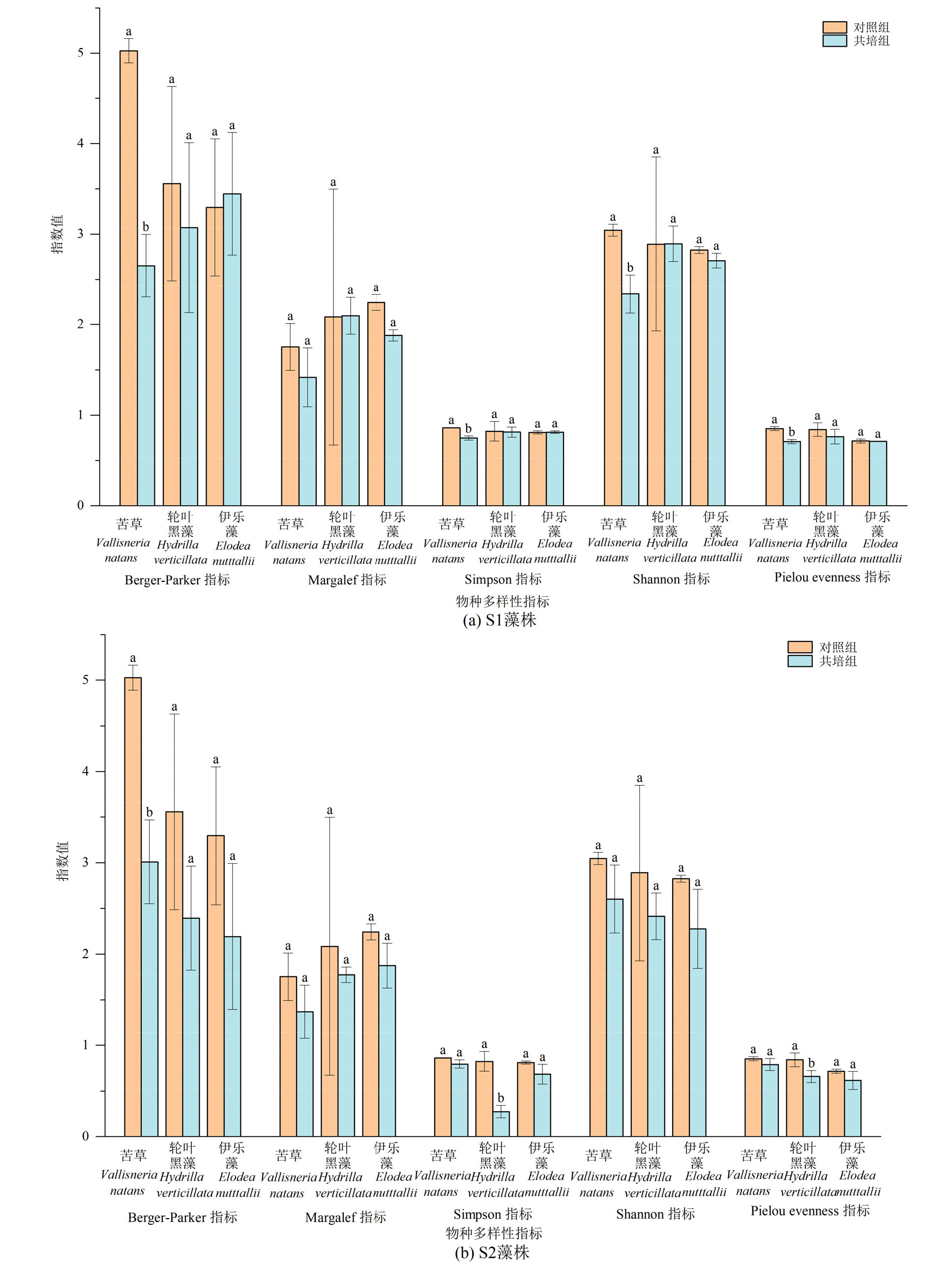
图7 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中周丛藻类多样性指数
Figure 7 Diversity indices of periphytic algae in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
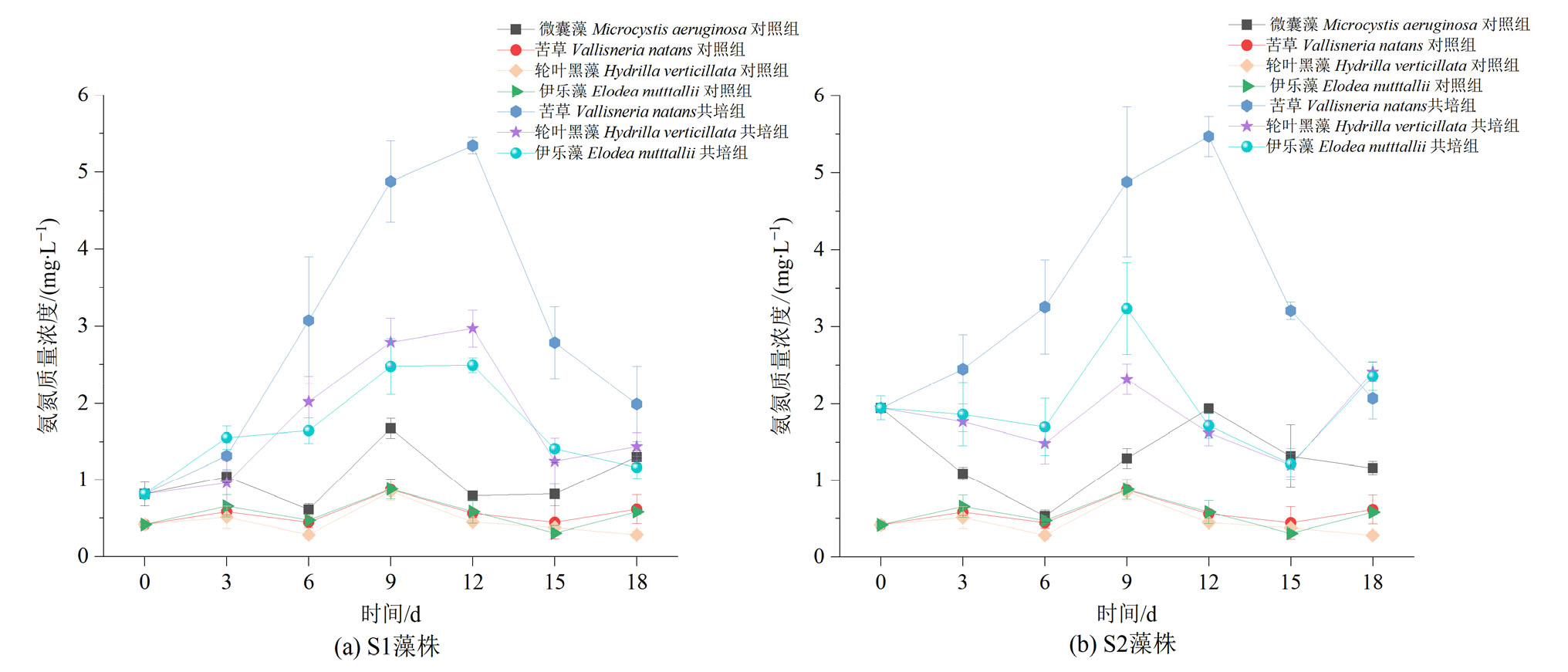
图8 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中氨氮质量浓度的变化动态
Figure 8 Dynamic changes in ammonia concentrations in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
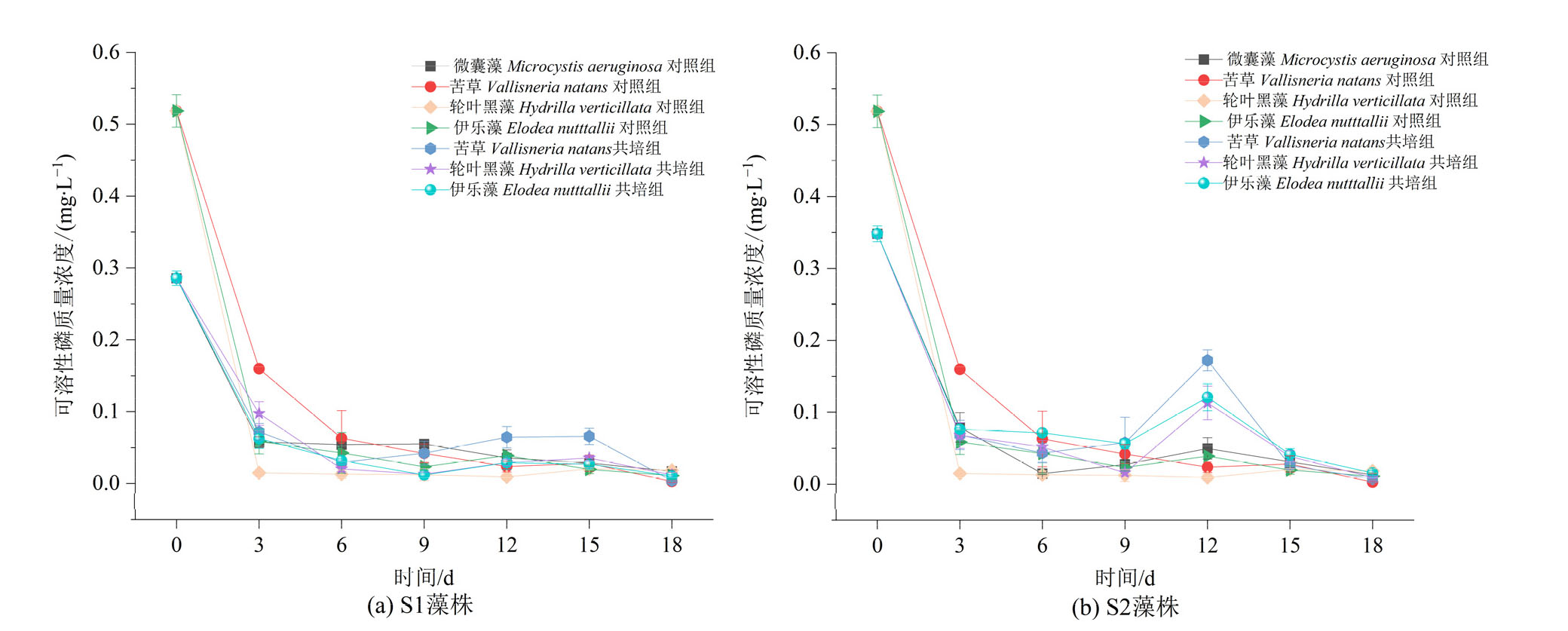
图9 苦草、轮叶黑藻和伊乐藻单培对照组及其与微囊藻共培组中可溶性磷含量的变化动态
Figure 9 Dynamic changes in phosphate concentrations in monoculture controls of V. natans, H. verticillata and E. nutttallii and co-culture groups with Microcystis
| [1] |
BAI G, ZHANG Y, YAN P, et al., 2020. Spatial and seasonal variation of water parameters, sediment properties, and submerged macrophytes after ecological restoration in a long-term (6 year) study in Hangzhou west lake in China: Submerged macrophyte distribution influenced by environmental variables[J]. Water Research, 186: 116379.
DOI URL |
| [2] |
DAVIS T W, GOBLER C J, 2016. Preface for special issue on “Global expansion of harmful cyanobacterial blooms: Diversity, ecology, causes, and controls”[J]. Harmful Algae, 54: 1-3.
DOI URL |
| [3] |
GAGET V, HUMPAGE A R, HUANG Q, et al., 2017. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs[J]. Water Research, 124: 454-464.
DOI PMID |
| [4] |
GAO Y N, GE F J, ZHANG L P, et al., 2017. Enhanced toxicity to the cyanobacterium Microcystis aeruginosa by low-dosage repeated exposure to the allelochemical N-phenyl-1-naphthylamine[J]. Chemosphere, 174: 732-738.
DOI URL |
| [5] |
GAO Y N, YANG H, GAO X F, et al., 2022. Ecological damage of submerged macrophyte Myriophyllum spicatum by cell extracts from microcystin (MC)- and non-MC-producing cyanobacteria, Microcystis [J]. Journal of Oceanology and Limnology, 40: 1732-1749.
DOI |
| [6] |
GAO Y N, YANG H, LI L F, et al., 2023. Higher resistance of a microcystin (MC)-producing cyanobacterium, Microcystis, to the submerged macrophyte Myriophyllum spicatum[J]. Environmental Science and Pollution Research, 30(23): 63941-63952.
DOI |
| [7] |
GUZZON A, BOHN A, DIOCIAIUTI M, et al., 2008. Cultured phototrophic biofilms for phosphorus removal in waste water treatment[J]. Water Research, 42(16): 4357-4367.
DOI URL |
| [8] |
GUBELIT Y I, GROSSART H P, 2020. New methods, new concepts: What can be applied to freshwater periphyton?[J]. Frontiers in Microbiology, 11: 1275.
DOI PMID |
| [9] |
HARKE M J, STEFFEN M M, GOBLER C J, et al., 2016. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp.[J]. Harmful Algae, 54: 4-20.
DOI URL |
| [10] |
HE Y, ZHOU Q H, LIU B.Y., et al., 2016. Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system[J]. Journal of Applied Phycology, 28(5): 2805-2814.
DOI URL |
| [11] |
JONES J I, SAYER C D, 2003. Does the fish-invertebrate-periphyton cascade precipitate plant loss in shallow lakes[J]. Ecology, 84(8): 2155-2167.
DOI URL |
| [12] |
MOHAMED Z A, AL SHEHRI A M, 2010. Differential responses of epiphytic and planktonic toxic cyanobacteria to allelopathic substances of the submerged macrophyte Stratiotes aloides[J]. International Review of Hydrobiology, 95(3): 224-234.
DOI URL |
| [13] | NICKLISCH A, KOHL J G, 1989. The influence of light on the primary production of two planktic blue-green algae[J]. Arch Hydrobiol Beih Ergebn Limnol, 33: 451-455. |
| [14] |
O’DRISCOLL C, EYTO D E, RODGERS M, et al., 2012. Diatom assemblages and their associated environmental factors in upland peat forest rivers[J]. Ecological Indicators, 18: 443-451.
DOI URL |
| [15] |
ROBERTS E, KROKER J, KÖRNER S, et al., 2003. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes[J]. Hydrobiologia, 506-509: 525-530.
DOI URL |
| [16] |
ROMO S, MIRACLE M R, VILLENA M J, et al., 2004. Mesocosm experiments on nutrient and fish effects on shallow lake food webs in a Mediterranean climate[J]. Freshwater Biology, 49(12): 1593-1607.
DOI URL |
| [17] |
WIJEWARDENE L, WU N, FOHRER N, et al., 2022. Epiphytic biofilms in freshwater and interactions with macrophytes: Current understanding and future directions[J]. Aquatic Botany, 176: 103467.
DOI URL |
| [18] |
WU Y H, HE J Z, YANG L, 2010. Evaluating adsorption and biodegradation mechanisms during the removal of Microcystin-RR by periphyton[J]. Environmental Science & Technology, 44(16): 6319-6324.
DOI URL |
| [19] |
WU Y, LIU J, YANG L, et al., 2011. Allelopathic control of cyanobacterial blooms by periphyton biofilms[J]. Environmental Microbiology, 13(3): 604-615.
DOI PMID |
| [20] | WU Z B, GAO Y N, WANG J, et al., 2009. Allelopathic effects of phenolic compounds present in submerged macrophytes on Microcystis aeruginosa [J]. Allelopathy Journal, 23(2): 403-410. |
| [21] |
SCHEFFER M, RINALDI S, GRAGNANI A, et al., 1997. On the dominance of filamentous cyanobacteria in shallow, turbid lakes[J]. Ecology, 78(1): 272-282.
DOI URL |
| [22] |
SOREN B, YVONNE V, PAUL S, 2016. Benthic algae compensate for phytoplankton losses in large aquatic ecosystems[J]. Global Change Biology, 22(12): 3865-3873.
DOI PMID |
| [23] | STEWART P M, PRATT J R, CAIRNS J, et al., 1985. Diatom and protozoan species accrual on artificial substrates in lentic habitats[J]. Microscopical Society, 104(4): 369-377. |
| [24] |
VADEBONCOEUR Y, STEINMAN A D, 2002. Periphyton function in lake ecosystems[J]. The Scientific World Journal, 2: 1449-1468.
DOI URL |
| [25] |
XIAO M, LI M, REYNOLDS C S, et al., 2018. Colony formation in the cyanobacterium Microcystis[J]. Biological Reviews, 93(3): 1399-1420.
DOI URL |
| [26] |
XU Y, WANG G X, YANG W B, et al., 2010. Dynamics of the water bloom-forming Microcystis and its relationship with physicochemical factors in Lake Xuanwu (China)[J]. Environmental Science and Pollution Research, 17(9): 1581-1590.
DOI URL |
| [27] |
YAN L Y, ZHANG S H, LIN D, et al., 2018. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands[J]. Science of The Total Environment, 622-623: 121-126.
DOI URL |
| [28] | YANG C T, SHEN X B, SHI X Y, et al., 2023. Impact of submerged macrophytes on growth and 2-MIB release risk of Pseudanabaena sp.: From field monitoring to cultural experiments[J]. Journal of Hazardous Materials, 442: 130052. |
| [29] |
YU J L, XIA M L, HE H, et al., 2021. Species-specific responses of submerged macrophytes to the presence of a small omnivorous bitterling Acheilognathus macropterus[J]. Science of the Total Environment, 753: 141998.
DOI URL |
| [30] |
ZHANG L, LIU B Y, GE F J, et al., 2019. Interspecific competition for nutrients between submerged macrophytes (Vallisneria natans, Ceratophyllum demersum) and filamentous green algae (Cladophora oligoclona) in a co-culture system[J]. Polish Journal of Environmental Studies, 28(3): 1483-1494.
DOI URL |
| [31] |
ZHANG L, PENG X, LIU B Y, et al., 2018. Effects of the decomposing liquid of Cladophora oligoclona on Hydrilla verticillata turion germination and seedling growth[J]. Ecotoxicology and Environmental Safety, 157: 81-88.
DOI URL |
| [32] |
ZHANG W Z, SHEN H, ZHANG J, et al., 2020. Physiological differences between free-floating and periphytic filamentous algae, and specific submerged macrophytes induce proliferation of filamentous algae: A novel implication for lake restoration[J]. Chemosphere, 239: 124702.
DOI URL |
| [33] |
ZHANG X F, LIU Z W, JEPPESEN E, et al., 2016. Effects of benthic-feeding common carp and filter-feeding silver carp on benthic-pelagic coupling: Implications for shallow lake management[J]. Ecological Engineering, 88: 256-264.
DOI URL |
| [34] |
ZHAO Y H, ZHANG Y, GUO J S, et al., 2023. Shifts in periphyton research themes over the past three decades[J]. Environmental Science and Pollution Research, 30(3): 5281-5295.
DOI |
| [35] | 胡鸿钧, 魏印心, 2006. 中国淡水藻类-系统、分类及生态[M]. 北京: 科学出版社. |
| HU H J, WEI Y X, 2009. Freshwater Algae in China- Systematics, Taxonomy and Ecology[M]. Beijing: Science Press. | |
| [36] | 何月, 2015. 底栖藻类在浅水湖泊稳态转换中的作用[D]. 武汉: 中南民族大学:39-40. |
| HE Y, 2015. The role of periphyton regime shifts in shallow lakes[D]. Wuhan: South-Central University for Nationalities:39-40. | |
| [37] | 邱东茹, 吴振斌, 刘保元, 1997. 武汉东湖水生植被的恢复试验研究[J]. 湖泊科学, 9(2): 168-174. |
|
QIU D R, WU Z B, LIU B Y, 1997. Ecological restoration of aquatic vegetation in a eutrophic shallow lake, Donghu lake, Wuhan[J]. Journal of Lake Sciences, 9(2): 168-174.
DOI URL |
|
| [38] | 尚媛媛, 2013. 蓝藻降解对大型沉水植物种群生长的影响[D]. 南京: 南京信息工程大学:18-19. |
| SHANG Y Y, 2013. The influence of cyanobacteria degradation on the submersed plant population growth[D]. Nanjing: Nanjing University of Information Science and Technology:18-19. | |
| [39] | 王立新, 2005. 黑藻对水华的抑制及其机制的研究[D]. 南京: 南京师范大学:30-31. |
| WANG L X, 2005. Study on the inhibition of water bloom by black algae and its mechanism[D]. Nanjing: Nanjing Normal University:30-31. | |
| [40] | 王涛, 2021. 富营养化水体中底栖藻类生长和沉水植物响应的机制研究[D]. 武汉: 中南民族大学:54-56. |
| WANG T, 2021. Study on the mechanism of benthic algae growth and submerged macrophytes response in eutrophic water[D]. Wuhan: South-Central Minzu University:54-56. | |
| [41] | 吴婷婷, 刘国锋, 韩士群, 等, 2015. 蓝藻水华聚集对水葫芦生理生态的影响[J]. 环境科学, 36(1): 114-120. |
| WU T T, LIU G F, HAN S Q, et al., 2015. Impacts of algal blooms accumulation on physiological ecology of water hyacinth[J]. Environmental Science, 36(1): 114-120. | |
| [42] | 吴振斌, 等, 2011. 水生植物与水体生态修复[M]. 北京: 科学出版社. |
| WU Z B, et al., 2011. Aquatic plant and ecological restoration of water environment[M]. Beijing: Science Press. | |
| [43] | 吴振斌, 邱东茹, 贺锋, 等, 2001. 水生植物对富营养水体水质净化作用研究[J]. 武汉植物学研究, 19(4): 299-303. |
| WU Z B, QIU D R, HE F, et al., 2001. Studies on eutrophicated water quality provement by means of Aquatic Macrophytes[J]. Journal of Wuhan Botanical Research, 19(4): 299-303. | |
| [44] | 张兵之, 2008. 伊乐藻对铜绿微囊藻的化感作用研究[D]. 武汉: 中国科学院研究生院(水生生物研究所):66-67. |
| ZHANG B Z, 2008. Studies on The Allelopathy of Elodea nuttallii on Microcystis aeruginosa [D]. Wuhan: Chinese Academy of Sciences (Institute of Hydrobiology): 66-67. |
| [1] | 童银栋, 黄兰兰, 杨宁, 张奕妍, 李子芃, 邵波. 全球水体微囊藻毒素分布特征及其潜在环境风险分析[J]. 生态环境学报, 2023, 32(1): 129-138. |
| [2] | 张孝进, 戴正为, 戴煜, 戴国飞, 杨平, 方媛瑗, 彭宁彦. 可同时控藻和除藻毒素的方法研究进展[J]. 生态环境学报, 2021, 30(7): 1549-1554. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
