生态环境学报 ›› 2023, Vol. 32 ›› Issue (4): 733-743.DOI: 10.16258/j.cnki.1674-5906.2023.04.011
王馨雨1( ), 高灯州1, 刘博林1, 王斌1, 郑艳玲2,3, 李小飞1, 侯立军1,*(
), 高灯州1, 刘博林1, 王斌1, 郑艳玲2,3, 李小飞1, 侯立军1,*( )
)
收稿日期:2023-01-14
出版日期:2023-04-18
发布日期:2023-07-12
通讯作者:
*侯立军(1975年生),男,教授,研究方向为河口海岸生源要素循环。E-mail: ljhou@sklec.ecnu.edu.cn作者简介:王馨雨(1997年生),女,硕士研究生,研究方向为河口海岸生源要素循环。E-mail: w_wwxy@163.com
基金资助:
WANG Xinyu1( ), GAO Dengzhou1, LIU Bolin1, WANG Bin1, ZHENG Yanling2,3, LI Xiaofei1, HOU Lijun1,*(
), GAO Dengzhou1, LIU Bolin1, WANG Bin1, ZHENG Yanling2,3, LI Xiaofei1, HOU Lijun1,*( )
)
Received:2023-01-14
Online:2023-04-18
Published:2023-07-12
摘要:
河口水体中硝化微生物的化能自养固碳(DCF)对碳氮循环过程有着重要影响,但目前关于河口水体氨氧化微生物对DCF过程的贡献鲜见报道。以长江口为研究区,利用14C和15N同位素示踪技术,分别测定了大潮和小潮期间水体DCF和硝化速率,并通过实时荧光定量PCR技术量化了相关功能基因丰度。结果表明,长江口水体大小潮期间,DCF和硝化速率分别介于170.72-1007.35 nmol?L?1?d?1和1.45-70.75 nmol?L?1?h?1,呈现大潮速率相对较高,小潮速率低的变化特征,且底层水体DCF和硝化速率显著高于表层水体。水体中铵盐和可溶性无机碳浓度是影响DCF和硝化速率的关键环境因子。定量PCR结果表明,大潮和小潮时cbbL基因丰度分别为0.40×108-3.40×108 copies?L?1和0.49×108-2.27×108 copies?L?1,均高于cbbM基因丰度(大潮:0.67×108-9.84×106 copies?L?1,小潮:0.75×108-5.73×106 copies?L?1)。小潮时水体accA基因丰度(0.16×108-2.65×108 copies?L?1)高于大潮时(0.20×108-3.92×108 copies?L?1),并且底层均高于表层。在整个潮周期中,自养固碳功能基因丰度总体呈现涨潮时增加,落潮时降低的变化趋势。氨氧化古菌(AOA)和氨氧化细菌(AOB)是DCF过程的主要贡献者,AOA amoA和AOB amoA丰度在大潮和小潮之间存在显著差异,大潮时AOA amoA基因丰度(0.22×107-3.59×107 copies?L?1)明显高于AOB amoA基因丰度(0.26×107-1.61×107 copies?L?1),而小潮时AOB amoA丰度占据优势,为0.92×106-1.32×106 copies?L?1,表明潮汐过程可通过改变水体化能自养微生物群落组成来影响DCF。该研究深化了长江口潮周期水体DCF和硝化过程速率变化特征的认识,揭示了河口水体硝化微生物驱动的DCF过程的重要性,以期为全球变化背景下河口生态系统碳汇功能评估提供一定的科学参考。
中图分类号:
王馨雨, 高灯州, 刘博林, 王斌, 郑艳玲, 李小飞, 侯立军. 长江口水体化能自养固碳过程的潮周期变化特征及影响因素[J]. 生态环境学报, 2023, 32(4): 733-743.
WANG Xinyu, GAO Dengzhou, LIU Bolin, WANG Bin, ZHENG Yanling, LI Xiaofei, HOU Lijun. The Tidal-cycle Variation and Influencing Factors of Dark Carbon Fixation Process in the Yangtze Estuary[J]. Ecology and Environment, 2023, 32(4): 733-743.
| 目标基因 | 引物名称 | 序列 (5′-3′) | 步骤及条件 | 参考文献 |
|---|---|---|---|---|
| Bacterial 16S rRNA gene | 341F | CCTACGGGAGGCAGCAG | 95 ℃ for 30 s, 45×[95 ℃ for 15 s, 59 ℃ for 30 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Muyzer et al., |
| 518R | ATTACCGCGGCTGCTGG | |||
| Archaea 16S rRNA gene | Arch967F | AATTGGCGGGGGAGCAC | 95 ℃ for 30 s, 40×[95 ℃ for 15 s, 60 ℃ for 1 min], 72 ℃ for 5 min | Cadillo-Quiroz et al., |
| Arch1060R | GGCCATGCACCWCCTCTC | |||
| Amo-AOA gene | Arch-amoAF | STAATGGTCTGGCTTAGACG | 50 ℃ for 2 min, 95 ℃ for 10 min, 45× [95 ℃ for 30s, 56 ℃ for 40s, 72 ℃ for 1 min], 72 ℃ for 5 min | Francis et al., |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | |||
| amoA-AOB gene | amoA-1F | GGGGTTTCTACTGGTGG | 50 ℃ for 2 min, 95 ℃ for 10 min, 45×[95 ℃ for 30 s, 58 ℃ for 40 s, 72 ℃ for 1 min], 72 ℃ for 5 min | Nicolaisen et al., |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | |||
| accA gene | Crena_529F | GCWATGACWGAYTTTGTYRTAATG | 95 ℃ for 30 s, 40× [95 ℃ for 5 s, 48 ℃ for 10 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Yakimov et al., |
| Crena_981R | TGGWTKRYTTGCAAYTATWCC | |||
| cbbM gene | cbbM343F | GGYAAYAACCARGGYATGGG | 95 ℃ for 30 s, 40×[95 ℃ for 5 s, 57 ℃ for 5 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Alfreider et al., |
| cbbM1126R | CGYARBGCRTTCATRCCRCC | |||
| cbbL gene | K2F | ACCAYCAAGCCSAAGCTSGG | 95 ℃ for 30 s, 40×[95 ℃ for 15 s, 63 ℃ for 1 min], 72 ℃ for 5 min | Nanba et al., |
| V2R | GCCTTCSAGCTTGCCSACCRC |
表1 qPCR的引物和条件
Table 1 Primers and thermal cycling conditions for qPCR
| 目标基因 | 引物名称 | 序列 (5′-3′) | 步骤及条件 | 参考文献 |
|---|---|---|---|---|
| Bacterial 16S rRNA gene | 341F | CCTACGGGAGGCAGCAG | 95 ℃ for 30 s, 45×[95 ℃ for 15 s, 59 ℃ for 30 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Muyzer et al., |
| 518R | ATTACCGCGGCTGCTGG | |||
| Archaea 16S rRNA gene | Arch967F | AATTGGCGGGGGAGCAC | 95 ℃ for 30 s, 40×[95 ℃ for 15 s, 60 ℃ for 1 min], 72 ℃ for 5 min | Cadillo-Quiroz et al., |
| Arch1060R | GGCCATGCACCWCCTCTC | |||
| Amo-AOA gene | Arch-amoAF | STAATGGTCTGGCTTAGACG | 50 ℃ for 2 min, 95 ℃ for 10 min, 45× [95 ℃ for 30s, 56 ℃ for 40s, 72 ℃ for 1 min], 72 ℃ for 5 min | Francis et al., |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | |||
| amoA-AOB gene | amoA-1F | GGGGTTTCTACTGGTGG | 50 ℃ for 2 min, 95 ℃ for 10 min, 45×[95 ℃ for 30 s, 58 ℃ for 40 s, 72 ℃ for 1 min], 72 ℃ for 5 min | Nicolaisen et al., |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | |||
| accA gene | Crena_529F | GCWATGACWGAYTTTGTYRTAATG | 95 ℃ for 30 s, 40× [95 ℃ for 5 s, 48 ℃ for 10 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Yakimov et al., |
| Crena_981R | TGGWTKRYTTGCAAYTATWCC | |||
| cbbM gene | cbbM343F | GGYAAYAACCARGGYATGGG | 95 ℃ for 30 s, 40×[95 ℃ for 5 s, 57 ℃ for 5 s, 72 ℃ for 34 s], 72 ℃ for 5 min | Alfreider et al., |
| cbbM1126R | CGYARBGCRTTCATRCCRCC | |||
| cbbL gene | K2F | ACCAYCAAGCCSAAGCTSGG | 95 ℃ for 30 s, 40×[95 ℃ for 15 s, 63 ℃ for 1 min], 72 ℃ for 5 min | Nanba et al., |
| V2R | GCCTTCSAGCTTGCCSACCRC |
| 样品 编号* | 温度/ ℃ | 盐度 | pH | ρ(DO)/ (mg∙L−1) | c(NH4+)/ (µmol∙L−1) | c(NO2−)/ (µmol∙L−1) | c(NO3−)/ (µmol∙L−1) | c(SiO32−)/ (µmol∙L−1) | c(PO43−)/ (µmol∙L−1) | c(DIC)/ (μmol∙L−1) | c(DOC)/ (mmol∙L−1) | c(TN)/ (µmol∙L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1S | 23.86 | 13.02 | 8.59 | 6.55 | 2.41±0.11 | 0.28±0.05 | 78.92±5.74 | 61.60±2.01 | 1.35±0.06 | 1534.58±38.53 | 1.47±0.08 | 86.52±4.24 |
| 1-1B | 23.62 | 13.05 | 8.59 | 5.98 | 3.05±0.18 | 0.24±0.03 | 75.54±3.62 | 58.66±1.59 | 1.17±0.09 | 1610.20±17.22 | 1.72±0.15 | 82.97±1.93 |
| 1-2S | 23.76 | 9.76 | 8.51 | 7.58 | 1.22±0.07 | 0.18±0.02 | 92.52±4.79 | 66.68±0.44 | 1.34±0.21 | 1566.72±33.60 | 1.71±0.10 | 107.58±3.45 |
| 1-2B | 23.61 | 10.54 | 8.55 | 6.64 | 1.57±0.12 | 0.20±0.01 | 88.95±5.11 | 67.97±4.12 | 1.31±0.12 | 1580.33±47.47 | 1.58±0.09 | 99.79±5.84 |
| 1-3S | 23.17 | 8.68 | 8.58 | 8.23 | 0.54±0.06 | 0.16±0.07 | 105.38±7.20 | 83.92±2.28 | 1.37±0.14 | 1529.29±71.58 | 1.67±0.11 | 126.88±2.02 |
| 1-3B | 23.48 | 9.02 | 8.50 | 6.91 | 1.32±0.12 | 0.22±0.02 | 96.78±6.58 | 73.02±1.75 | 1.36±0.12 | 1544.03±17.71 | 1.67±0.13 | 110.21±6.59 |
| 1-4S | 23.35 | 9.08 | 8.51 | 6.27 | 1.58±0.09 | 0.18±0.04 | 87.47±6.02 | 74.91±5.62 | 1.28±0.10 | 1540.63±8.49 | 1.54±0.09 | 103.51±0.34 |
| 1-4B | 23.26 | 11.44 | 8.51 | 6.13 | 2.24±0.20 | 0.21±0.03 | 87.65±5.83 | 70.99±1.26 | 1.20±0.28 | 1567.21±68.35 | 1.70±0.14 | 96.65±5.69 |
| 1-5S | 22.93 | 12.42 | 8.52 | 6.45 | 1.74±0.17 | 0.28±0.01 | 85.66±6.77 | 75.78±4.35 | 1.12±0.19 | 1497.53±33.12 | 1.55±0.12 | 94.46±1.80 |
| 1-5B | 22.89 | 15.67 | 8.46 | 6.28 | 3.14±0.29 | 0.33±0.02 | 73.52±7.09 | 61.42±2.40 | 1.12±0.10 | 1580.71±20.80 | 1.57±0.08 | 80.37±2.02 |
| 1-6S | 23.41 | 10.88 | 8.47 | 6.35 | 1.44±0.13 | 0.22±0.06 | 83.16±6.92 | 75.37±2.51 | 1.32±0.07 | 1533.07±55.28 | 1.57±0.09 | 100.96±0.44 |
| 1-6B | 23.44 | 10.76 | 8.48 | 6.35 | 1.53±0.12 | 0.26±0.02 | 88.70±4.45 | 75.34±2.09 | 1.34±0.09 | 1562.18±38.46 | 1.50±0.13 | 97.47±2.05 |
| 1-7S | 23.48 | 10.65 | 8.49 | 6.51 | 0.73±0.11 | 0.26±0.04 | 94.85±7.06 | 79.85±2.13 | 1.35±0.16 | 1475.23±10.55 | 1.52±0.08 | 107.66±2.49 |
| 1-7B | 23.52 | 10.89 | 8.46 | 6.28 | 1.31±0.10 | 0.28±0.01 | 91.46±4.92 | 76.26±1.20 | 1.33±0.05 | 1567.10±0.85 | 1.65±0.23 | 110.86±1.53 |
| 1-8S | 23.65 | 13.89 | 8.44 | 6.05 | 2.30±0.14 | 0.26±0.02 | 82.30±10.31 | 73.66±1.78 | 1.31±0.02 | 1531.94±101.79 | 1.38±0.14 | 93.34±5.63 |
| 1-8B | 23.89 | 11.92 | 8.47 | 6.09 | 2.23±0.19 | 0.30±0.03 | 87.19±6.39 | 74.72±3.53 | 1.26±0.09 | 1536.09±40.64 | 1.49±0.11 | 98.38±3.58 |
| 2-1S | 23.46 | 9.88 | 8.28 | 8.15 | 1.15±0.13 | 0.31±0.01 | 76.78±9.07 | 85.79±2.11 | 1.38±0.04 | 1451.03±67.00 | 1.38±0.10 | 105.35±2.71 |
| 2-1B | 23.31 | 10.32 | 8.46 | 7.02 | 1.73±0.14 | 0.35±0.02 | 85.28±6.52 | 81.68±2.89 | 1.31±0.08 | 1491.48±22.21 | 1.49±0.09 | 106.17±6.44 |
| 2-2S | 21.80 | 9.94 | 8.19 | 8.15 | 0.63±0.09 | 0.35±0.03 | 87.88±5.03 | 84.90±4.03 | 1.20±0.12 | 1399.23±21.89 | 1.52±0.05 | 105.97±2.05 |
| 2-2B | 23.07 | 10.34 | 8.44 | 6.81 | 1.26±0.10 | 0.38±0.11 | 88.04±6.88 | 80.71±3.70 | 1.21±0.05 | 1488.08±54.67 | 1.88±0.12 | 100.05±5.58 |
| 2-3S | 23.87 | 8.16 | 8.21 | 6.88 | 0.90±0.06 | 0.32±0.01 | 89.46±3.12 | 85.09±2.76 | 1.42±0.03 | 1438.93±1.44 | 1.53±0.09 | 113.93±10.52 |
| 2-3B | 23.57 | 8.56 | 8.42 | 6.83 | 1.13±0.07 | 0.35±0.04 | 91.67±5.68 | 82.62±4.74 | 1.32±0.25 | 1461.61±30.53 | 1.58±0.13 | 114.02±1.31 |
| 2-4S | 23.23 | 7.83 | 8.41 | 6.72 | 1.46±0.11 | 0.42±0.03 | 92.93±3.82 | 83.89±3.22 | 1.26±0.12 | 1434.02±30.63 | 1.84±0.20 | 147.64±7.21 |
| 2-4B | 23.15 | 8.21 | 8.49 | 6.54 | 0.83±0.05 | 0.35±0.01 | 94.04±6.43 | 84.03±3.58 | 1.30±0.14 | 1449.14±57.88 | 1.58±0.02 | 113.58±6.09 |
| 2-5S | 23.65 | 7.76 | 8.35 | 6.41 | 1.10±0.09 | 0.31±0.02 | 94.78±4.19 | 74.34±4.11 | 0.90±0.05 | 1436.28±44.07 | 1.54±0.40 | 107.81±0.34 |
| 2-5B | 23.45 | 10.89 | 8.45 | 6.17 | 2.24±0.24 | 0.36±0.02 | 95.54±5.60 | 74.34±2.59 | 1.24±0.06 | 1492.24±10.85 | 1.71±0.09 | 103.18±8.88 |
| 2-6S | 23.60 | 8.90 | 8.32 | 6.40 | 0.81±0.05 | 0.30±0.05 | 96.87±2.10 | 80.94±2.03 | 1.25±0.11 | 1423.81±19.24 | 1.42±0.11 | 110.62±2.12 |
| 2-6B | 23.42 | 11.01 | 8.44 | 6.21 | 1.78±0.13 | 0.39±0.02 | 97.19±7.21 | 74.36±1.89 | 1.25±0.10 | 1458.21±76.73 | 1.44±0.10 | 105.64±3.79 |
| 2-7S | 24.35 | 7.66 | 8.36 | 6.59 | 0.58±0.05 | 0.35±0.08 | 97.50±5.16 | 84.82±3.55 | 1.35±0.04 | 1393.94±39.09 | 1.41±0.05 | 106.61±6.02 |
| 2-7B | 24.34 | 7.37 | 8.31 | 6.77 | 0.65±0.05 | 0.34±0.04 | 97.81±4.45 | 81.03±5.23 | 1.32±0.16 | 1443.47±13.23 | 1.54±0.14 | 110.45±1.89 |
| 2-8S | 24.21 | 6.88 | 8.29 | 6.80 | 0.25±0.01 | 0.27±0.03 | 105.61±8.92 | 87.07±4.24 | 1.37±0.06 | 1418.89±70.02 | 1.62±0.29 | 123.13±4.33 |
| 2-8B | 24.21 | 7.09 | 8.30 | 6.76 | 0.94±0.04 | 0.30±0.01 | 107.37±10.39 | 84.92±2.36 | 1.35±0.12 | 1437.66±11.18 | 1.61±0.11 | 117.18±6.69 |
表2 采样点理化特征
Table 2 Physical and chemical characteristics of sampling stations
| 样品 编号* | 温度/ ℃ | 盐度 | pH | ρ(DO)/ (mg∙L−1) | c(NH4+)/ (µmol∙L−1) | c(NO2−)/ (µmol∙L−1) | c(NO3−)/ (µmol∙L−1) | c(SiO32−)/ (µmol∙L−1) | c(PO43−)/ (µmol∙L−1) | c(DIC)/ (μmol∙L−1) | c(DOC)/ (mmol∙L−1) | c(TN)/ (µmol∙L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1S | 23.86 | 13.02 | 8.59 | 6.55 | 2.41±0.11 | 0.28±0.05 | 78.92±5.74 | 61.60±2.01 | 1.35±0.06 | 1534.58±38.53 | 1.47±0.08 | 86.52±4.24 |
| 1-1B | 23.62 | 13.05 | 8.59 | 5.98 | 3.05±0.18 | 0.24±0.03 | 75.54±3.62 | 58.66±1.59 | 1.17±0.09 | 1610.20±17.22 | 1.72±0.15 | 82.97±1.93 |
| 1-2S | 23.76 | 9.76 | 8.51 | 7.58 | 1.22±0.07 | 0.18±0.02 | 92.52±4.79 | 66.68±0.44 | 1.34±0.21 | 1566.72±33.60 | 1.71±0.10 | 107.58±3.45 |
| 1-2B | 23.61 | 10.54 | 8.55 | 6.64 | 1.57±0.12 | 0.20±0.01 | 88.95±5.11 | 67.97±4.12 | 1.31±0.12 | 1580.33±47.47 | 1.58±0.09 | 99.79±5.84 |
| 1-3S | 23.17 | 8.68 | 8.58 | 8.23 | 0.54±0.06 | 0.16±0.07 | 105.38±7.20 | 83.92±2.28 | 1.37±0.14 | 1529.29±71.58 | 1.67±0.11 | 126.88±2.02 |
| 1-3B | 23.48 | 9.02 | 8.50 | 6.91 | 1.32±0.12 | 0.22±0.02 | 96.78±6.58 | 73.02±1.75 | 1.36±0.12 | 1544.03±17.71 | 1.67±0.13 | 110.21±6.59 |
| 1-4S | 23.35 | 9.08 | 8.51 | 6.27 | 1.58±0.09 | 0.18±0.04 | 87.47±6.02 | 74.91±5.62 | 1.28±0.10 | 1540.63±8.49 | 1.54±0.09 | 103.51±0.34 |
| 1-4B | 23.26 | 11.44 | 8.51 | 6.13 | 2.24±0.20 | 0.21±0.03 | 87.65±5.83 | 70.99±1.26 | 1.20±0.28 | 1567.21±68.35 | 1.70±0.14 | 96.65±5.69 |
| 1-5S | 22.93 | 12.42 | 8.52 | 6.45 | 1.74±0.17 | 0.28±0.01 | 85.66±6.77 | 75.78±4.35 | 1.12±0.19 | 1497.53±33.12 | 1.55±0.12 | 94.46±1.80 |
| 1-5B | 22.89 | 15.67 | 8.46 | 6.28 | 3.14±0.29 | 0.33±0.02 | 73.52±7.09 | 61.42±2.40 | 1.12±0.10 | 1580.71±20.80 | 1.57±0.08 | 80.37±2.02 |
| 1-6S | 23.41 | 10.88 | 8.47 | 6.35 | 1.44±0.13 | 0.22±0.06 | 83.16±6.92 | 75.37±2.51 | 1.32±0.07 | 1533.07±55.28 | 1.57±0.09 | 100.96±0.44 |
| 1-6B | 23.44 | 10.76 | 8.48 | 6.35 | 1.53±0.12 | 0.26±0.02 | 88.70±4.45 | 75.34±2.09 | 1.34±0.09 | 1562.18±38.46 | 1.50±0.13 | 97.47±2.05 |
| 1-7S | 23.48 | 10.65 | 8.49 | 6.51 | 0.73±0.11 | 0.26±0.04 | 94.85±7.06 | 79.85±2.13 | 1.35±0.16 | 1475.23±10.55 | 1.52±0.08 | 107.66±2.49 |
| 1-7B | 23.52 | 10.89 | 8.46 | 6.28 | 1.31±0.10 | 0.28±0.01 | 91.46±4.92 | 76.26±1.20 | 1.33±0.05 | 1567.10±0.85 | 1.65±0.23 | 110.86±1.53 |
| 1-8S | 23.65 | 13.89 | 8.44 | 6.05 | 2.30±0.14 | 0.26±0.02 | 82.30±10.31 | 73.66±1.78 | 1.31±0.02 | 1531.94±101.79 | 1.38±0.14 | 93.34±5.63 |
| 1-8B | 23.89 | 11.92 | 8.47 | 6.09 | 2.23±0.19 | 0.30±0.03 | 87.19±6.39 | 74.72±3.53 | 1.26±0.09 | 1536.09±40.64 | 1.49±0.11 | 98.38±3.58 |
| 2-1S | 23.46 | 9.88 | 8.28 | 8.15 | 1.15±0.13 | 0.31±0.01 | 76.78±9.07 | 85.79±2.11 | 1.38±0.04 | 1451.03±67.00 | 1.38±0.10 | 105.35±2.71 |
| 2-1B | 23.31 | 10.32 | 8.46 | 7.02 | 1.73±0.14 | 0.35±0.02 | 85.28±6.52 | 81.68±2.89 | 1.31±0.08 | 1491.48±22.21 | 1.49±0.09 | 106.17±6.44 |
| 2-2S | 21.80 | 9.94 | 8.19 | 8.15 | 0.63±0.09 | 0.35±0.03 | 87.88±5.03 | 84.90±4.03 | 1.20±0.12 | 1399.23±21.89 | 1.52±0.05 | 105.97±2.05 |
| 2-2B | 23.07 | 10.34 | 8.44 | 6.81 | 1.26±0.10 | 0.38±0.11 | 88.04±6.88 | 80.71±3.70 | 1.21±0.05 | 1488.08±54.67 | 1.88±0.12 | 100.05±5.58 |
| 2-3S | 23.87 | 8.16 | 8.21 | 6.88 | 0.90±0.06 | 0.32±0.01 | 89.46±3.12 | 85.09±2.76 | 1.42±0.03 | 1438.93±1.44 | 1.53±0.09 | 113.93±10.52 |
| 2-3B | 23.57 | 8.56 | 8.42 | 6.83 | 1.13±0.07 | 0.35±0.04 | 91.67±5.68 | 82.62±4.74 | 1.32±0.25 | 1461.61±30.53 | 1.58±0.13 | 114.02±1.31 |
| 2-4S | 23.23 | 7.83 | 8.41 | 6.72 | 1.46±0.11 | 0.42±0.03 | 92.93±3.82 | 83.89±3.22 | 1.26±0.12 | 1434.02±30.63 | 1.84±0.20 | 147.64±7.21 |
| 2-4B | 23.15 | 8.21 | 8.49 | 6.54 | 0.83±0.05 | 0.35±0.01 | 94.04±6.43 | 84.03±3.58 | 1.30±0.14 | 1449.14±57.88 | 1.58±0.02 | 113.58±6.09 |
| 2-5S | 23.65 | 7.76 | 8.35 | 6.41 | 1.10±0.09 | 0.31±0.02 | 94.78±4.19 | 74.34±4.11 | 0.90±0.05 | 1436.28±44.07 | 1.54±0.40 | 107.81±0.34 |
| 2-5B | 23.45 | 10.89 | 8.45 | 6.17 | 2.24±0.24 | 0.36±0.02 | 95.54±5.60 | 74.34±2.59 | 1.24±0.06 | 1492.24±10.85 | 1.71±0.09 | 103.18±8.88 |
| 2-6S | 23.60 | 8.90 | 8.32 | 6.40 | 0.81±0.05 | 0.30±0.05 | 96.87±2.10 | 80.94±2.03 | 1.25±0.11 | 1423.81±19.24 | 1.42±0.11 | 110.62±2.12 |
| 2-6B | 23.42 | 11.01 | 8.44 | 6.21 | 1.78±0.13 | 0.39±0.02 | 97.19±7.21 | 74.36±1.89 | 1.25±0.10 | 1458.21±76.73 | 1.44±0.10 | 105.64±3.79 |
| 2-7S | 24.35 | 7.66 | 8.36 | 6.59 | 0.58±0.05 | 0.35±0.08 | 97.50±5.16 | 84.82±3.55 | 1.35±0.04 | 1393.94±39.09 | 1.41±0.05 | 106.61±6.02 |
| 2-7B | 24.34 | 7.37 | 8.31 | 6.77 | 0.65±0.05 | 0.34±0.04 | 97.81±4.45 | 81.03±5.23 | 1.32±0.16 | 1443.47±13.23 | 1.54±0.14 | 110.45±1.89 |
| 2-8S | 24.21 | 6.88 | 8.29 | 6.80 | 0.25±0.01 | 0.27±0.03 | 105.61±8.92 | 87.07±4.24 | 1.37±0.06 | 1418.89±70.02 | 1.62±0.29 | 123.13±4.33 |
| 2-8B | 24.21 | 7.09 | 8.30 | 6.76 | 0.94±0.04 | 0.30±0.01 | 107.37±10.39 | 84.92±2.36 | 1.35±0.12 | 1437.66±11.18 | 1.61±0.11 | 117.18±6.69 |
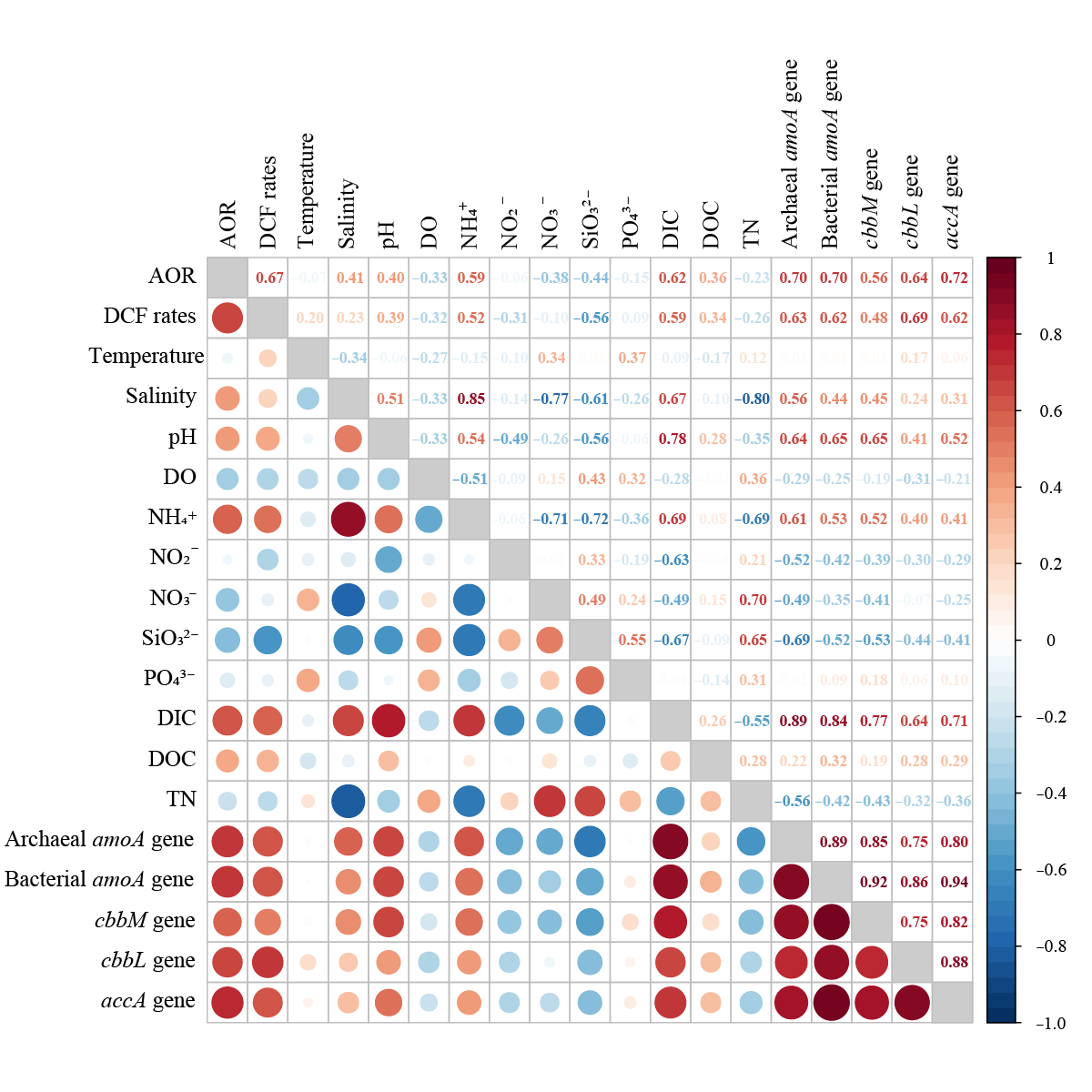
图6 化能自养固碳速率、硝化速率、功能基因丰度和理化特征之间的相关性 数字代表皮尔逊相关系数。红色代表正相关;蓝色代表负相关;颜色的深浅和圆圈的大小代表相关性的强度
Figure 6 Correlations among DCF rates, functional gene abundances, and physicochemical characteristics
| [1] |
ALFREIDER A, BAUMER A, BOGENSPERGER T, et al., 2017. CO2 assimilation strategies in stratified lakes: Diversity and distribution patterns of chemolithoautotrophs[J]. Environmental Microbiology, 19(7): 2754-2768.
DOI URL |
| [2] |
ALFREIDER A, VOGT C, HOFFMANN D, et al., 2003. Diversity of ribulose-1, 5-bisphosphate carboxylase/oxygenase large-subunit genes from groundwater and aquifer microorganisms[J]. Microbial Ecology, 45(4): 317-328.
DOI URL |
| [3] |
ANDERSSON M G I, BRION N, MIDDELBURG J J, 2006. Comparison of nitrifier activity versus growth in the Scheldt estuary-a turbid, tidal estuary in northern Europe[J]. Aquatic Microbial Ecology, 42(2): 149-158.
DOI URL |
| [4] |
BADGER M R, BEK E J, 2008. Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle[J]. Journal of Experimental Botany, 59(7): 1525-1541.
DOI URL |
| [5] | BARBOZA C D N, PAES E T, DE ANDRADE JANDRE K, et al., 2014. Concentrations and fluxes of nutrients and suspended organic matter in a tropical estuarine system: The Tinharé-Boipeba, Islands Archipelago (Baixo Sul Baiano, Brazil)[J]. Journal of Coastal Research, 30(6): 1197-1209. |
| [6] |
BAUER J E, CAI W J, RAYMOND P A, et al., 2013. The changing carbon cycle of the coastal ocean[J]. Nature, 504: 61-70.
DOI |
| [7] | BERG C, LISTMANN L, VANDIEKEN V, et al., 2015. Chemoautotrophic growth of ammonia-oxidizing Thaumarchaeota enriched from a pelagic redox gradient in the Baltic Sea[J]. Frontiers in Microbiology, 5: 786. |
| [8] |
BERG I A, 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways[J]. Applied and Environmental Microbiology, 77(6): 1925-1936.
DOI URL |
| [9] |
BERG I A, KOCKELKORN D, RAMOS-VERA W H, et al., 2010. Autotrophic carbon fixation in archaea[J]. Nature Reviews Microbiology, 8(6): 447-460.
DOI PMID |
| [10] |
BERGAUER K, SINTES E, BLEIJSWIJK J, et al., 2013. Abundance and distribution of archaeal acetyl-CoA/propionyl-CoA carboxylase genes indicative for putatively chemoautotrophic Archaea in the tropical Atlantic’s interior[J]. FEMS Microbiology Ecology, 84(3): 461-473.
DOI URL |
| [11] |
BRÄUER S L, KRANZLER K, GOODSON N, et al., 2013. Dark carbon fixation in the Columbia River’s Estuarine turbidity maxima: Molecular characterization of red-Type cbbL genes and measurement of DIC uptake rates in response to added electron donors[J]. Estuaries and Coasts, 36(5): 1073-1083.
DOI URL |
| [12] |
CADILLO-QUIROZ H, BRÄUER S, YASHIRO E, et al., 2006. Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA[J]. Environmental Microbiology, 8(8): 1428-1440.
DOI URL |
| [13] |
CAI W J, 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration?[J]. Annual Review of Marine Science, 3(1): 123-145.
DOI URL |
| [14] |
CASAMAYOR E O, GARCÍACANTIZANO J, MAS J, et al., 2001. Primary production in estuarine oxic/anoxic interfaces: contribution of microbial dark CO2 fixation in the Ebro River Salt Wedge Estuary[J]. Marine Ecology Progress, 215(1): 49-56.
DOI URL |
| [15] |
CASCIOTTI K L, SIGMAN D M, HASTINGS M G, et al., 2002. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method[J]. Analytical Chemistry, 74(19): 4905-4912.
PMID |
| [16] |
DAI Z J, DU J Z, ZHANG X L, et al., 2011. Variation of riverine material loads and environmental consequences on the Changjiang (Yangtze) Estuary in recent decades (1955-2008)[J]. Environmental Science and Technology, 45(1): 223-227.
DOI PMID |
| [17] |
DAIMS H, LEBEDEVA E V, PJEVAC P, et al., 2015. Complete nitrification by Nitrospira bacteria[J]. Nature, 528(7583): 504-509.
DOI |
| [18] |
DAIMS H, LÜCKER S, WAGNER M, et al., 2016. A new perspective on microbes formerly known as Nitrite-Oxidizing Bacteria[J]. Trends in Microbiology, 24(9): 699-712.
DOI PMID |
| [19] |
DANG H, ZHOU H, YANG J, et al., 2013. Thaumarchaeotal signature gene distribution in sediments of the Northern South China Sea: an indicator of the metabolic intersection of the marine carbon, nitrogen, and phosphorus cycles?[J]. Applied and Environmental Microbiology, 79(7): 2137-2147.
DOI URL |
| [20] |
DELONG E, HALLAM S, MINCER T, et al., 2006. Correction: Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine crenarchaeota[J]. PLoS Biology, 4(12): e437.
DOI URL |
| [21] |
DUARTE C M, LOSADA I J, HENDRIKS I E, et al., 2013. The role of coastal plant communities for climate change mitigation and adaptation[J]. Nature Climate Change, 3(11): 961-968.
DOI |
| [22] |
FISTAROL G O, COUTINHO F H, MOREIRA A P, et al., 2015. Environmental and sanitary conditions of Guanabara Bay, Rio de Janeiro[J]. Frontiers in Microbiology, 6: 1232.
DOI PMID |
| [23] |
FORREST W W, WALKER D J, et al., 1971. The generation and utilization of energy during growth[J]. Advances in Microbial Physiology, 5: 213-274.
PMID |
| [24] |
FRANCIS C A, ROBERTS K J, BEMAN J M, et al., 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean[J]. Proceedings of the National Academy of Sciences, 102(41): 14683-14688.
DOI URL |
| [25] |
FRANKENBACH S, EZEQUIEL J, PLECHA S, et al., 2020. Synoptic spatio-temporal variability of the photosynthetic productivity of microphytobenthos and phytoplankton in a tidal estuary[J]. Frontiers in Marine Science, 7: 170.
DOI URL |
| [26] |
GONZÁLEZ J M, FERNÁNDEZ-GÓMEZ B, FERNÀNDEZ-GUERRA A, et al., 2008. Genome analysis of the proteorhodopsin-containing marine bacterium Polaribacter sp MED152 (Flavobacteria)[J]. Proceedings of the National Academy of Sciences, 105(25): 8724-8729.
DOI URL |
| [27] |
GUERRERO-FEIJÓO E, E SINTES, HERNDL G J, et al., 2018. High dark inorganic carbon fixation rates by specific microbial groups in the Atlantic off the Galician coast (NW Iberian margin)[J]. Environmental Microbiology, 20(2): 602-611.
DOI URL |
| [28] |
HERNDL G J, REINTHALER T, TEIRA E, et al., 2005. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean[J]. Applied and Environmental Microbiology, 71(5): 2303-2309.
PMID |
| [29] |
HERRMANN M, RUSZNYÁK A, AKOB D M, et al., 2015. Large fractions of CO2-fixing microorganisms in pristine limestone aquifers appear to be involved in the oxidation of reduced sulfur and nitrogen compounds[J]. Applied and Environmental Microbiology, 81(7): 2384-2394.
DOI PMID |
| [30] |
HU H W, MACDONALD CA, TRIVEDI P, et al., 2016. Effects of climate warming and elevated CO2 on autotrophic nitrification and nitrifiers in dryland ecosystems[J]. Soil Biology and Biochemistry, 92: 1-15.
DOI URL |
| [31] |
KÖNNEKE M, BERNHARD A E, DE LA TORRE J R, et al., 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon[J]. Nature: 437(7058): 543-546.
DOI |
| [32] |
LIANG C, 2020. Soil microbial carbon pump: Mechanism and appraisal[J]. Soil Ecology Letters, 2(4): 241-254.
DOI |
| [33] |
LONG X E, YAO H, WANG J, et al., 2015. Community structure and soil pH determine chemoautotrophic carbon dioxide fixation in drained paddy soils[J]. Environmental Science and Technology, 49(12): 7152-7160.
DOI URL |
| [34] |
MANTOURA R F C, WOODWARD E M S, 1983. Optimization of the indophenol blue method for the automated determination of ammonia in estuarine waters. Estuarine[J]. Estuarine, Coastal and Shelf Science, 17(2): 219-224.
DOI URL |
| [35] |
MENG W Q, FEAGIN R A, HU B B, et al., 2019. The spatial distribution of blue carbon in the coastal wetlands of China[J]. Estuarine Coastal and Shelf Science, 222: 13-20.
DOI |
| [36] | MIDDELBURG J J, 2011. Chemoautotrophy in the ocean[J]. Geophysical Research Letters, 38(24): 94-97. |
| [37] |
MUYZER G, DE WAAL E C, UITTERLINDEN A G, et al., 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA[J]. Applied and Environmental Microbiology, 59(3): 695-700.
DOI URL |
| [38] |
NANBA K, KING G M, DUNFIELD K, 2004. Analysis of facultative lithotroph distribution and diversity on volcanic deposits by use of the large subunit of ribulose 1, 5-bisphosphate carboxylase/oxygenase[J]. Applied and Environmental Microbiology, 70(4): 2245-2253.
DOI URL |
| [39] | NEWELL S E, BABBIN A R, JAYAKUMAR A, et al., 2011. Ammonia oxidation rates and nitrification in the Arabian Sea[J]. Global Biogeochemical Cycles, 25(4): 003940. |
| [40] |
NICOLAISEN M H, RAMSING N B, 2002. Denaturing gradient gel electrophoresis (DGGE) approaches to study the diversity of ammonia-oxidizing bacteria[J]. Journal of Microbiological Methods, 50(2): 189-203.
PMID |
| [41] |
PACHIADAKI M G, SINTES E, BERGAUER K, et al., 2017. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation[J]. Science, 358(6366): 1046-1051.
DOI PMID |
| [42] |
PENG X, FUCHSMAN C A, JAYAKUMAR A, et al., 2015. Ammonia and nitrite oxidation in the Eastern Tropical North Pacific[J]. Global Biogeochemical Cycles, 29(12): 2034-2049.
DOI URL |
| [43] |
PERNER M, PETERSEN J M, ZIELINSKI F, et al., 2010. Geochemical constraints on the diversity and activity of H2-oxidizing microorganisms in diffuse hydrothermal fluids from a basalt- and an ultramafic-hosted vent[J]. FEMS Microbiology Ecology, 74(1): 55-71.
DOI URL |
| [44] | PJEVAC P, DYKSMA S, GOLDHAMMER T, et al., 2019. In situ abundance and carbon fixation activity of distinct anoxygenic phototrophs in the stratified seawater lake Rogoznica[J]. Cold Spring Harbor Laboratory, 21(10): 3896-3908. |
| [45] | QIN W, AMIN SA, MARTENS-HABBENA W, et al., 2014. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation[J]. PANS, 111(34): 12504-12509. |
| [46] |
RIBAS-RIBAS M, ANFUSO E, GÓMEZ-PARRA A, et al., 2013. Tidal and seasonal carbon and nutrient dynamics of the Guadalquivir estuary and the Bay of Cádiz (SW Iberian Peninsula)[J]. Biogeosciences, 10(7): 4481-4491.
DOI URL |
| [47] |
SCHUERCH M, SPENCER T, TEMMERMAN S, et al., 2018. Future response of global coastal wetlands to sea-level rise[J]. Nature, 561(7722): 231-234.
DOI |
| [48] |
SIGMAN D M, CASCIOTTI K L, ANDREANI M, et al., 2001. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater[J]. Analytical Chemistry, 73(17): 4145-4153.
PMID |
| [49] |
SIMONA C, VENTURI S, TASSI F, et al., 2022. Geochemical and microbiological profiles in hydrothermal extreme acidic environments (Pisciarelli Spring, Campi Flegrei, Italy)[J]. FEMS microbiology ecology, 98(10): fiac088.
DOI URL |
| [50] |
STEWART B M, ELLIOTT P A W, 1996. Systematic salt effects in the automated determination of nutrients in seawater[J]. Water Research, 30(4): 869-874.
DOI URL |
| [51] |
STORELLI N, PEDUZZI S, SAAD M M, et al., 2013. CO2 assimilation in the chemocline of Lake Cadagno is dominated by a few types of phototrophic purple sulfur bacteria[J]. FEMS Microbiology Ecology, 84(2): 421-432.
DOI URL |
| [52] |
TAY H W, BRYAN K R, PILDITCH C A, et al., 2012. Variations in nutrient concentrations at different time scales in two shallow tidally dominated estuaries[J]. Marine and Freshwater Research, 63(2): 95-109.
DOI URL |
| [53] |
TAYLOR G T, WAY J, SCRANTON M I, 2003. Planktonic carbon cycling and transport in surface waters of the highly urbanized Hudson River estuary[J]. Limnology and Oceanography, 48(5): 1779-1795.
DOI URL |
| [54] |
TUTTLE J H, JANNASCH H W, 1977. Thiosulfate stimulation of microbial dark assimilation of carbon dioxide in shallow marine waters[J]. Microbial Ecology, 4(1): 9-25.
DOI PMID |
| [55] |
VAN KESSEL M A H, SPETH D R, ALBERTSEN M, et al., 2015. Complete nitrification by a single microorganism[J]. Nature, 528(7583): 555-559.
DOI |
| [56] |
VASQUEZ-CARDENAS D, MEYSMAN F, BOSCHKER H, 2020. A cross-system comparison of dark carbon fixation in coastal sediments[J]. Global Biogeochemical Cycles, DOI: 10.1029/2019GB006298.
DOI |
| [57] |
WARD B B, 2011. Measurement and Distribution of Nitrification Rates in the Oceans[J]. Methods in Enzymology, 486: 307-323.
DOI PMID |
| [58] |
WRIGHTON K C, CASTELLE C J, VARALJAY V A, et al., 2016. RubisCO of a nucleoside pathway known from Archaea is found in diverse uncultivated phyla in bacteria[J]. The ISME Journal, 10(11): 2702-2714.
DOI |
| [59] |
XIA W, BOWATTE S, JIA Z, et al., 2022. Offsetting N2O emissions through nitrifying CO2 fixation in grassland soil[J]. Soil Biology and Biochemistry, DOI: 10.1016/j.soilbio.2021.108528.
DOI |
| [60] |
YAKIMOV M M, CONO V L, DENARO R, 2009. A first insight into the occurrence and expression of functional amoA and accA genes of autotrophic and ammonia-oxidizing bathypelagic Crenarchaeota of Tyrrhenian Sea[J]. Deep Sea Research Part II Topical Studies in Oceanography, 56(11-12): 748-754.
DOI URL |
| [61] |
YAKIMOV M M, CONO V L, SMEDILE F, 2014. Heterotrophic bicarbonate assimilation is the main process of de novo organic carbon synthesis in hadal zone of the Hellenic Trench, the deepest part of Mediterranean Sea[J]. Environmental Microbiology Reports, 6(6): 709-722.
PMID |
| [62] |
ZHANG Y, QIN W, HOU L, et al., 2020. Nitrifier adaptation to low energy flux controls inventory of reduced nitrogen in the dark ocean[J]. Proceedings of the National Academy of Sciences, 117(9): 4823-4830.
DOI URL |
| [63] | ZHAO Y, LIU P, RUI J, et al., 2020. Dark carbon fixation and chemolithotrophic microbial community in surface sediments of the cascade reservoirs, Southwest China[J]. The Science of the Total Environment, 34(2): e2019GB006298. |
| [64] | ZHENG Z Z, WAN X, XU M N, et al., 2017. Effects of temperature and particles on nitrification in a eutrophic coastal bay in southern China[J]. Journal of Geophysical Research, 122(9): 2325-2337. |
| [65] |
ZHOU W, LIAO J, GUO Y, et al., 2017. High dark carbon fixation in the tropical South China Sea[J]. Continental Shelf Research, 146: 82-88.
DOI URL |
| [66] | 艾威, 李茂田, 刘晓强, 等, 2018. 长江口南槽最大浑浊带枯季大小潮悬沙峰特征及其动力机制[J]. 海洋与湖沼, 49(4): 769-778. |
| AI W, LI M T, LIU X Q, et al., 2018. Hydrodynamics of ssc peak in dry season of the south passage of changjiang river estuary[J]. Oceanologia Et Limnologia Sinica, 49(4): 769-778 | |
| [67] | 刘琼, 魏晓梦, 吴小红, 等, 2017. 稻田土壤固碳功能微生物群落结构和数量特征[J]. 环境科学, 38(2): 760-768. |
| LIU Q, WEI X M, WU X H, et al., 2017. Characteristic of abundances and diversity of carbon dioxide fixation microbes in paddy soils[J]. Environmental Science, 38(2): 760-768. |
| [1] | 薛力园, 刘志亮, 宋伟, 安颖, 袁晓博, 陈晓. 秦皇岛海域春季海月水母碟状幼体空间分布及其与海洋环境因子的关系[J]. 生态环境学报, 2021, 30(6): 1240-1248. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
